Supramolecular polymer and application thereof in fluorescent recognition of hydroxide ions and carbon dioxide gas
A technology for supramolecular polymers and fluorescent recognition, applied in the field of supramolecular polymers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
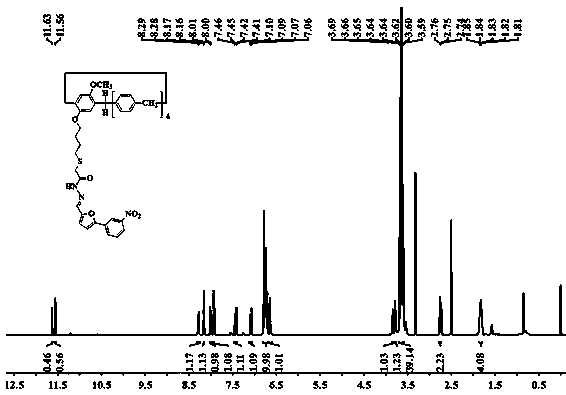
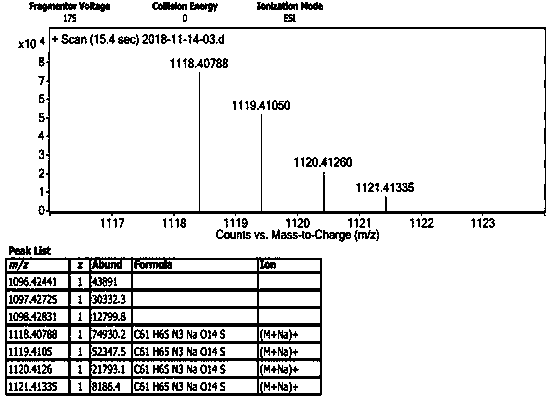
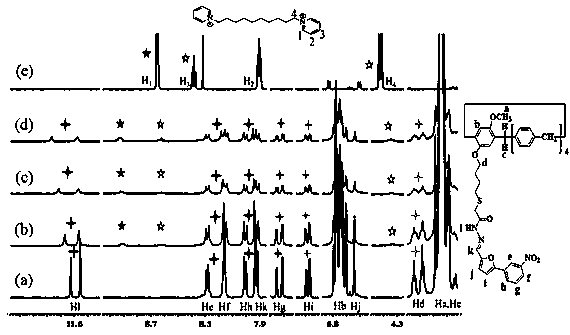
[0054] Embodiment 1, the synthesis of supramolecular polymer PNG
[0055] 1. Preparation of pillar arene derivative PN
[0056] Preparation of compound 1: under nitrogen protection, 4-methoxyphenol (2.48 g, 20.0 mmol), K 2 CO 3 (13.82 g, 100 mmol), KI (3.32 g, 20 mmol), 1,4-dibromobutane (17.12 g, 80 mmol) in acetone (400 mL) was refluxed for 48h. Filter after completion of the reaction, and the filtrate column chromatography (V 石油醚 / V 乙酸乙酯 = 50:1), to obtain white solid compound 1 (4.95 g, yield 96%);
[0057] Preparation of compound 2: Take compound 1 (1.29 g, 5 mmol), 1,4-dimethoxybenzene (8.29 g, 60 mmol), boron trifluoride ether (6 mL, 47.6 mmol) and paraformaldehyde ( 3.00 g, 100 mmol) was stirred in 1,2-dichloroethane (250 mL) at 30°C for 40 min. After the completion of the reaction, the mixed solution was washed with water and extracted 3 times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, and column chromatography (...
Embodiment 2
[0063] Embodiment 2. Recognition OH of supramolecular polymer PNG -
[0064] Pipette 2 mL PNG in DMSO solution (C PN =2×10 -4 M) Add 5 times the equivalent of F to a series of colorimetric tubes - , Cl - , Br - , I - , AcO - , H 2 PO 4 - , HSO 4 - , ClO 4 - , CN - , SCN - , N 3 - and OH - aqueous solution (C=2mM), if the fluorescence of the DMSO solution of PNG is quenched, it means that OH is added - , if the fluorescence of the PNG solution does not change, it means that the addition is not OH - .
Embodiment 3
[0065] Embodiment 3, the identification CO of supramolecular polymer PNG 2 gas
[0066] Pipette 2 ml PNG in DMSO (C PNG =2×10 -4 M), and add 5 equivalents of OH - of (C OH - =2mM) aqueous solution to obtain PNG-OH solution, exposed to the air, if the color of the solution becomes lighter after 5 minutes, and the solution completely changes from red to yellow after 10 minutes, indicating that there is CO in the air 2 Gas exists; if exposed to air for 10 minutes, the fluorescence of the solution turns on, indicating the presence of CO 2 gas.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com