Dihydrazone compound high in affinity with Abeta protein and Tau protein, derivative thereof, and applications of dihydrazone compound and derivative
An amide compound and compound technology, which is applied in the field of medical imaging, can solve the problems of low signal-to-noise ratio, poor in vivo stability, unable to be practically applied, slow removal speed, etc., and achieves high specificity, good pharmacokinetic properties, and rapidity. clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
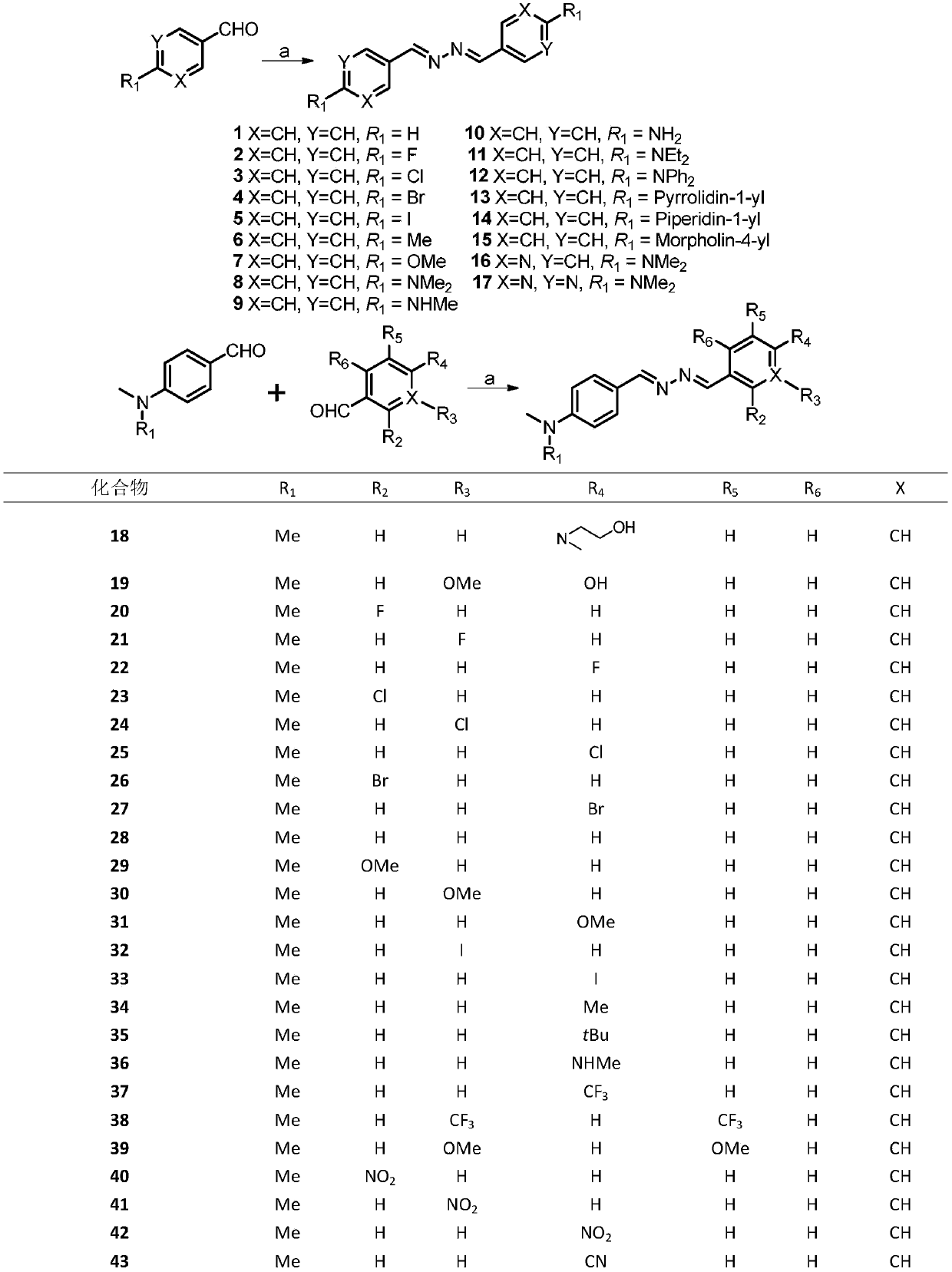
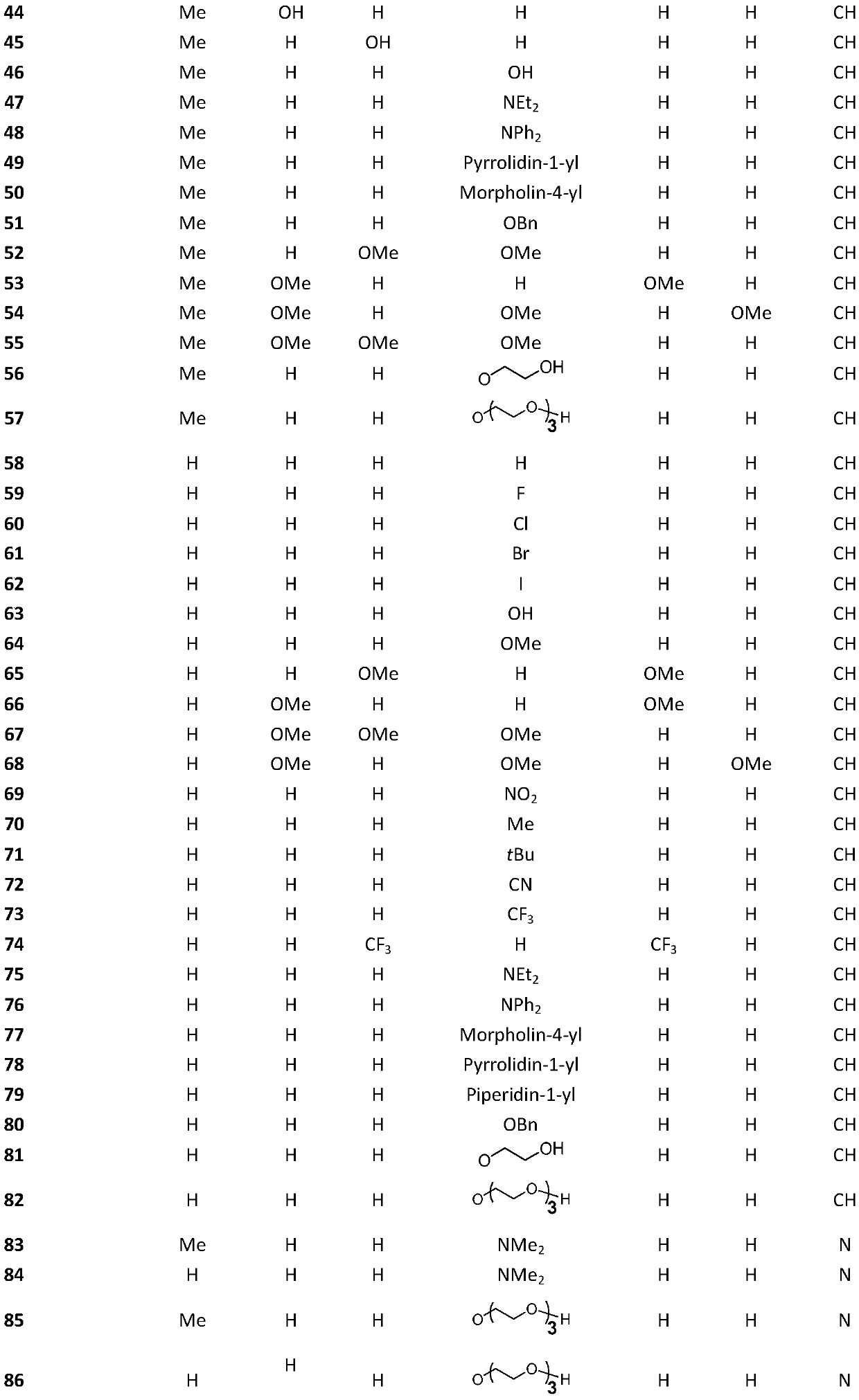
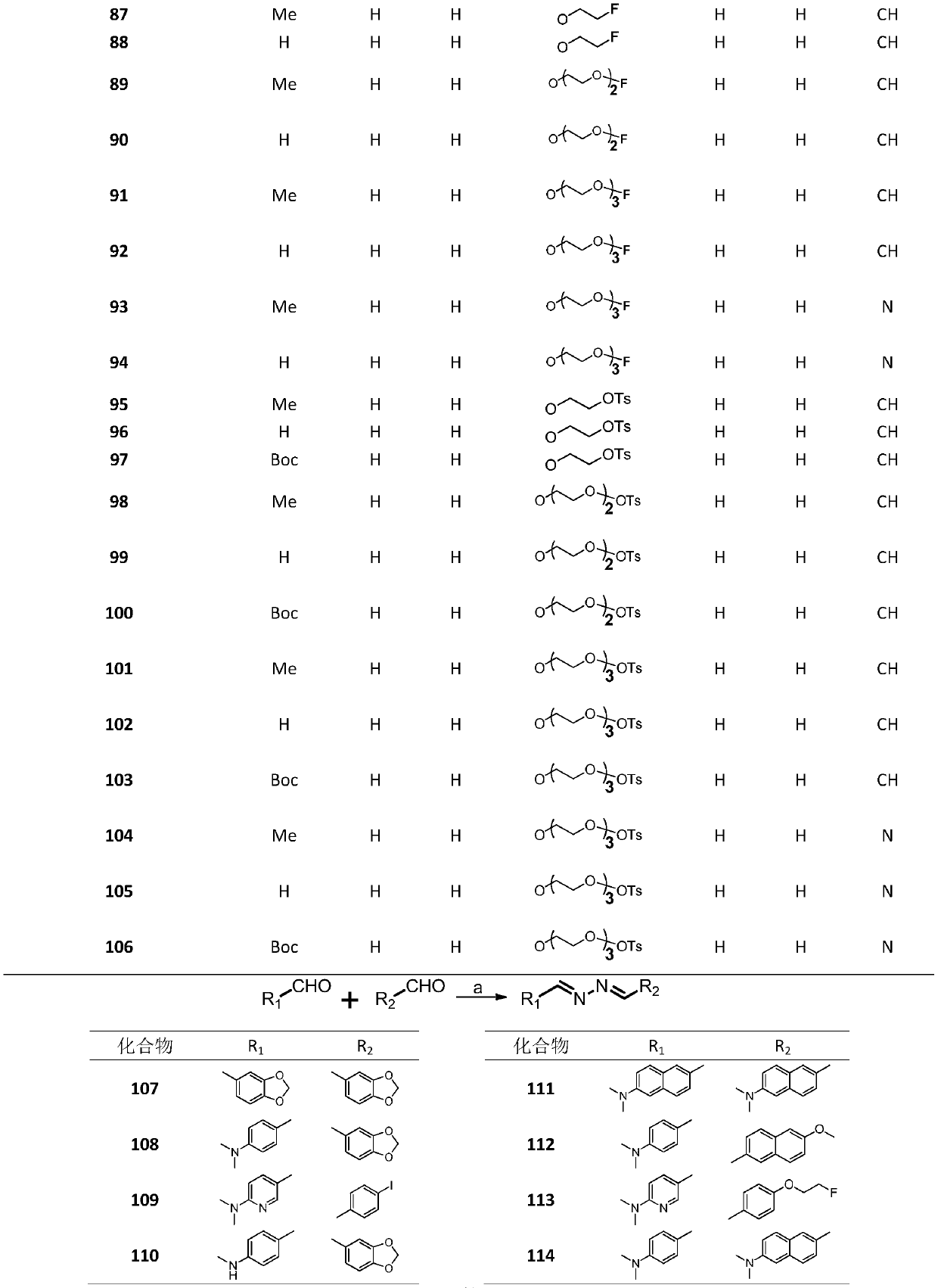
[0029] Embodiment 1: synthetic compound 1
[0030]
[0031] The compound benzaldehyde (212.4mg, 2.0mmol) was dissolved in 10mL ethanol in a 50mL round bottom flask, then hydrazine hydrate (58.9mg, 1.0mmol) was slowly added to the reaction flask, and the reaction was refluxed at 90°C for 10 minutes. After the reaction was completed, , cooled until a yellow solid precipitated, the precipitated product was suction filtered, and washed with 10 mL of ethanol and petroleum ether, and the crystalline product obtained by suction filtration was dried to obtain 44.0 mg of compound 1, with a yield of 18.5%, and the structure is as follows : 1 H NMR (400MHz, CDCl 3 )δ8.68 (s, 2H), 7.86 (dd, J=6.5, 3.0Hz, 4H), 7.56–7.35 (m, 6H).
Embodiment 2
[0032] Embodiment 2: synthetic compound 2
[0033]
[0034] Compound 2 was prepared from p-fluorobenzaldehyde according to the method for synthesizing compound 1, and 190.0 mg of a milky white solid was obtained with a yield of 77.8%. The structure is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.62(s,2H),7.90–7.74(m,4H),7.21–7.06(m,4H).
Embodiment 3
[0035] Embodiment 3: synthetic compound 3
[0036]
[0037] Compound 3 was prepared from 4-chlorobenzaldehyde according to the method for synthesizing compound 1, and 131.0 mg of light yellow crystalline solid was obtained with a yield of 47.3%. The structure was as follows: 1 H NMR (400MHz, CDCl 3 )δ8.61(s,2H),7.78(d,J=8.5Hz,4H),7.43(d,J=8.5Hz,4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com