Tricyclic compound as well as preparation method and application thereof
A compound and hydrate technology, applied in the field of medicinal chemistry, can solve problems such as druggability, safety and effectiveness hidden dangers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
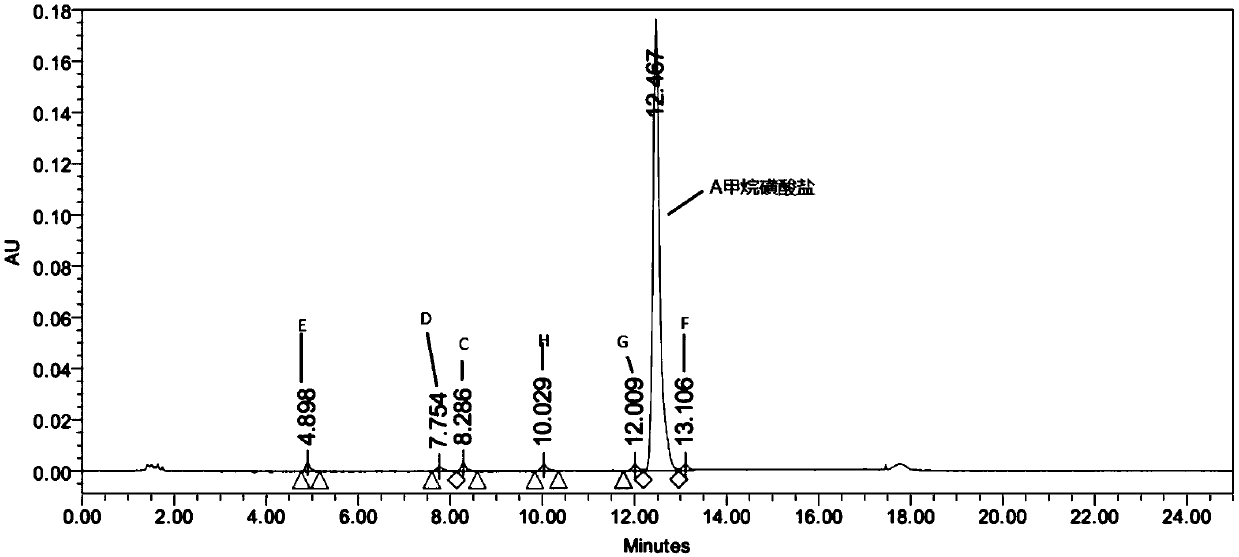
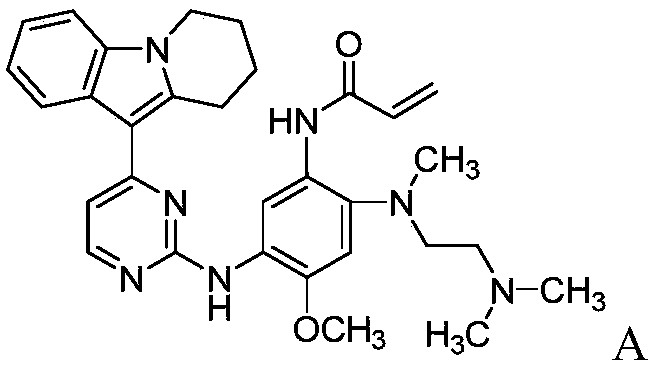
[0060] Example 1N-(2-((2-(dimethylamino)ethyl)(methyl)amino)-4-methoxy-5-((4-(6,7,8,9-tetrahydro Preparation of pyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)amino)phenyl)acrylamide methanesulfonate
[0061]
[0062] Step 1: Synthesis of 10-(2-chloropyrimidin-4-yl)-6,7,8,9-tetrahydropyrido[1,2-a]indole
[0063]
[0064] In a 100L vertical jacketed glass reactor, add ethylene glycol dimethyl ether (39.15kg) and 2,4-dichloropyrimidine (3.915kg), cool the solid-liquid mixture to below 10°C, and add anhydrous Aluminum chloride (3.855kg), the rate of addition is controlled, and the temperature is not higher than 30°C. After the addition is complete, stir at 25±5°C for 30 minutes, then add 6,7,8,9-tetrahydropyrido[1,2-a]indole (4.500kg), heat up, and react 3 at 60±5°C After 1 hour, the HPLC monitors that the content of 6,7,8,9-tetrahydropyrido[1,2-a]indole does not exceed 1.0%, and the reaction is determined to be complete. Cool the reaction solution below 25°C, add purified wate...
Embodiment 2
[0080] Example 2N-(5-acrylamido-4-((2-(dimethylamino)ethyl)methyl)amino)-2-methoxyphenyl)-N-(4-(6,7 ,8,9-Tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)acrylamide
[0081]
[0082] Add N to the 250mL three-neck flask 1 -(2-(Dimethylamino)ethyl)-5-methoxy-N 1 -Methyl-N 4 -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)benzene-1,2,4-triamine (1.60g , 3.3mmol, 1.0eq), N,N-dimethylacetamide (16mL), acryloyl chloride (0.90g, 9.9mmol, 3.0eq), N,N-diisopropylethylamine (2.13g, 16.5mmol , 5.0eq), the mixture was stirred at room temperature for 1.5 hours. 50 mL of methanol was added to the reaction mixture to precipitate a solid, which was filtered and dried in vacuo to obtain the title compound, 0.60 g in total, with a yield of 30.0%. 1 HNMR (300MHz, DMSO-d 6 )δ10.03(s,1H),8.59(d,J=5.5Hz,1H),8.19(s,1H),7.91(d,J=8.0Hz,1H),7.45-7.42(m,2H), 7.17(d,J=7.0Hz,1H),7.09-7.06(m,2H),6.77(dd,J 1 =17.0Hz,J 2 =10.3Hz,1H),6.42(dd,J 1 =17.0Hz,J 2 =10.0Hz,1H),6.29(d,J=1...
Embodiment 3
[0083] Example 3N-(4-methoxy-2-(methylamino)-5-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indole-10- Base) pyrimidin-2-yl) amino) phenyl) acrylamide
[0084]
[0085] Step 1: 2-Methoxy-N 4 -Methyl-5-nitro-N 1 Preparation of -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidin-2-yl)phenyl-1,4-diamine
[0086]
[0087] N-(4-fluoro-2-methoxy-5-nitrophenyl)-4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl ) pyrimidine-2-amine (6.00g, 13.8mmol, 1.0eq), methylamine tetrahydrofuran solution (50mL, 100mmol, 7.2eq) and N,N-dimethylacetamide (60mL) were added to the reaction flask, stirred React overnight at room temperature. Work up the next day yielded 5.50 g of the title compound.
[0088] Step 2: (5-methoxy-2-nitro-4-((4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyrimidine- Preparation of 2-yl)amino)phenyl)(methyl)carbamate tert-butyl ester
[0089]
[0090] Get the 2-methoxy-N that step 1 makes 4 -Methyl-5-nitro-N 1 -(4-(6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl)pyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com