Method of producing a sodium iron(II)-hexacyanoferrate(II) material
A technology of hexacyanoferrate, iron acid, applied in chemical instruments and methods, ferricyanide, iron compounds, etc., to achieve the effect of minimizing energy cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
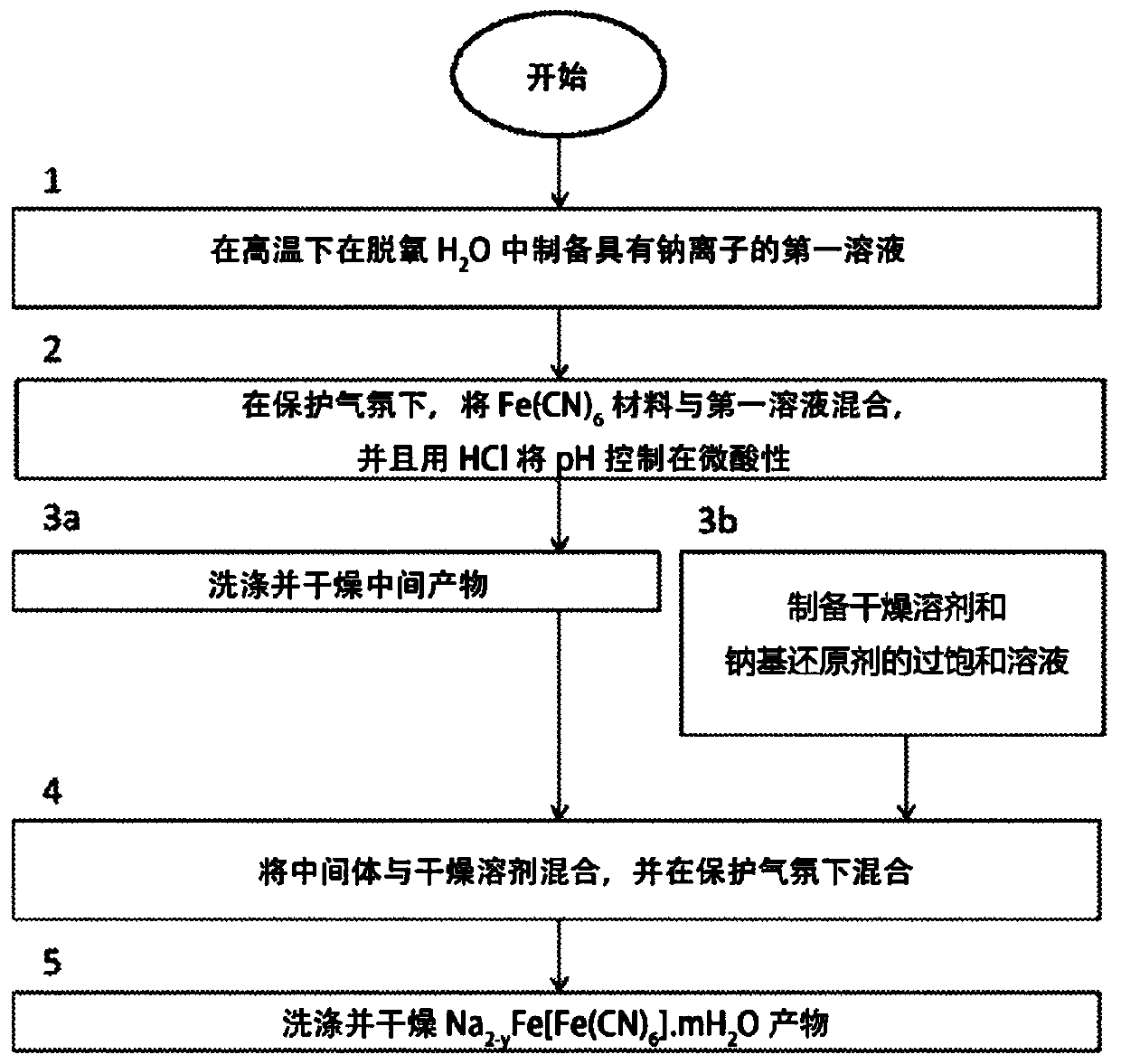
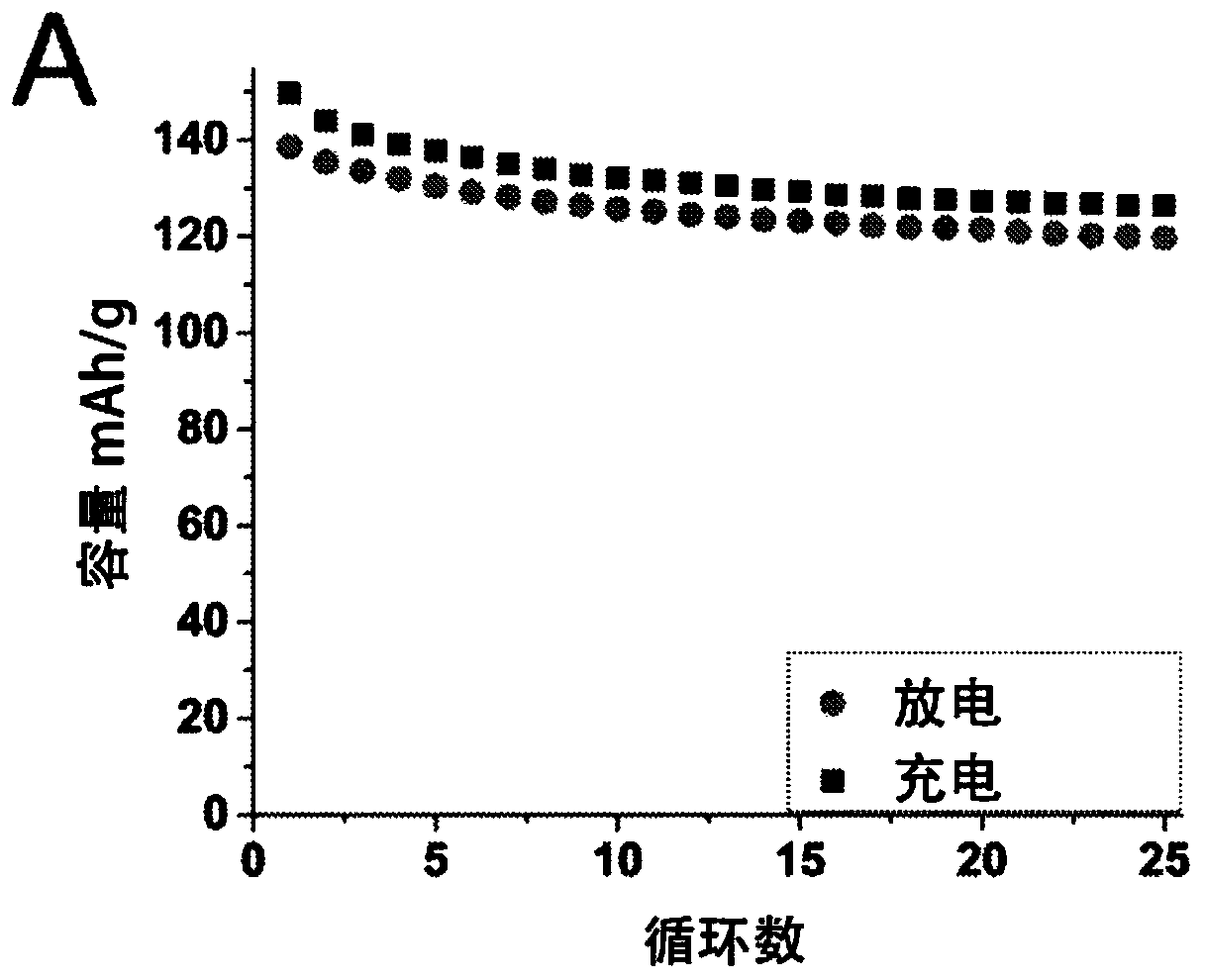
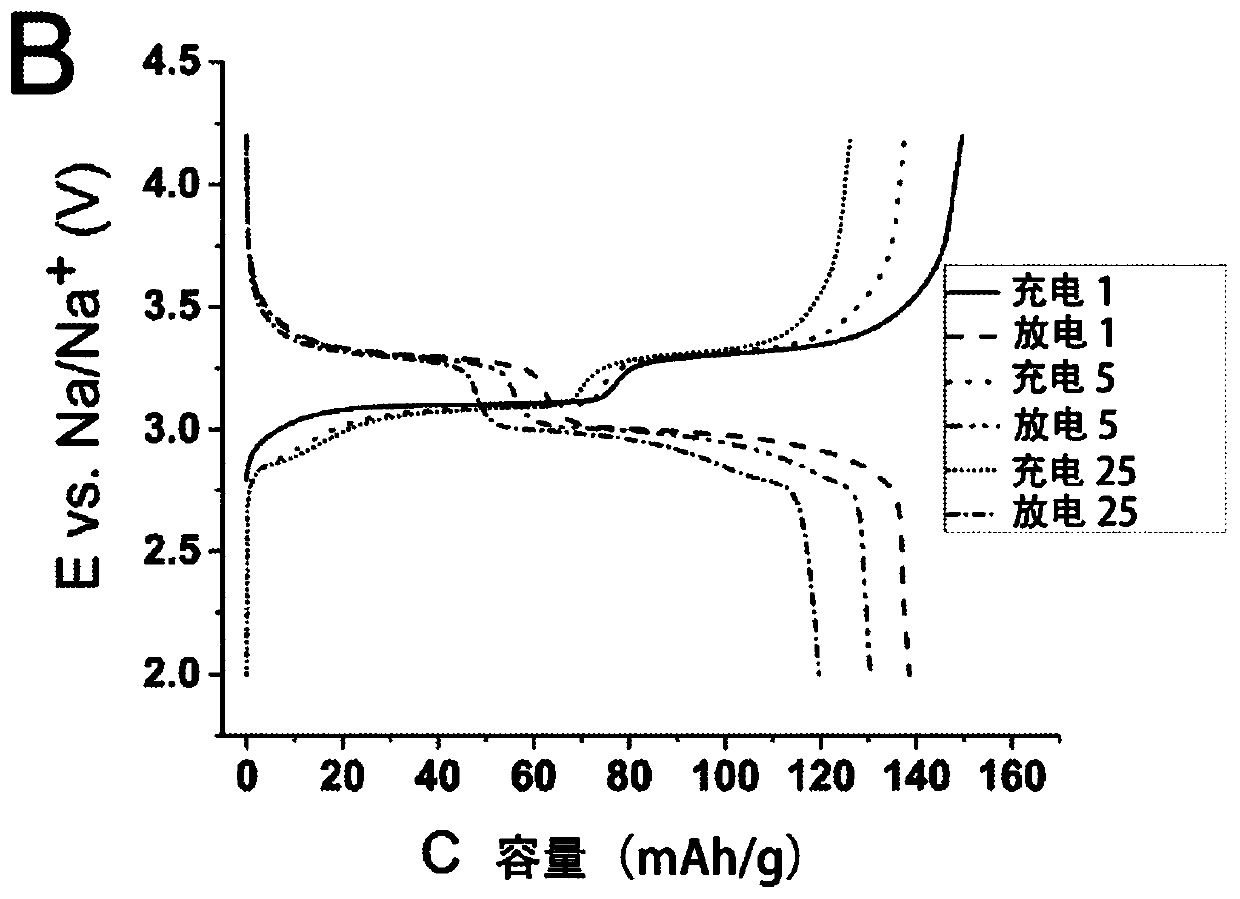
[0041] Production of ferric (II) sodium (Na) hexacyanoferrate (II) according to the present invention 2-y Fe[Fe(CN) 6 ].mH 2 O where y is less than x and preferably less than 0.2) method comprising two stages: (A) Na 4 Fe(CN) 6 The acid decomposes and dries into powder material, and (B) the sodium content of enriched powder material, Na 2-x Fe[Fe(CN) 6 ].mH 2 O, where x<0.4. The method according to the invention for producing a positive electrode for a sodium battery comprises a further stage (C) of forming an electrode comprising a sodium-enriched powder material.
[0042] The first stage (A) of the method according to the invention comprises Na 4 Fe(CN) 6 .10H 2 Acid decomposition of O, which is known in the art. However, it is a significant aspect that the chemical reactions taking place during the method according to the invention are carried out below 100°C and at or near ambient pressure. The reaction starts with Na 4 Fe(CN) 6 .10H 2 Acid decomposition of O...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com