Sterile and exquisite process of pharmaceutical raw materials
A technology for pharmaceuticals and original drugs, which is applied in the field of sterilization and refined technology of pharmaceutical originals, to achieve the effects of ensuring sterilization, ensuring dryness and internal cleanliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
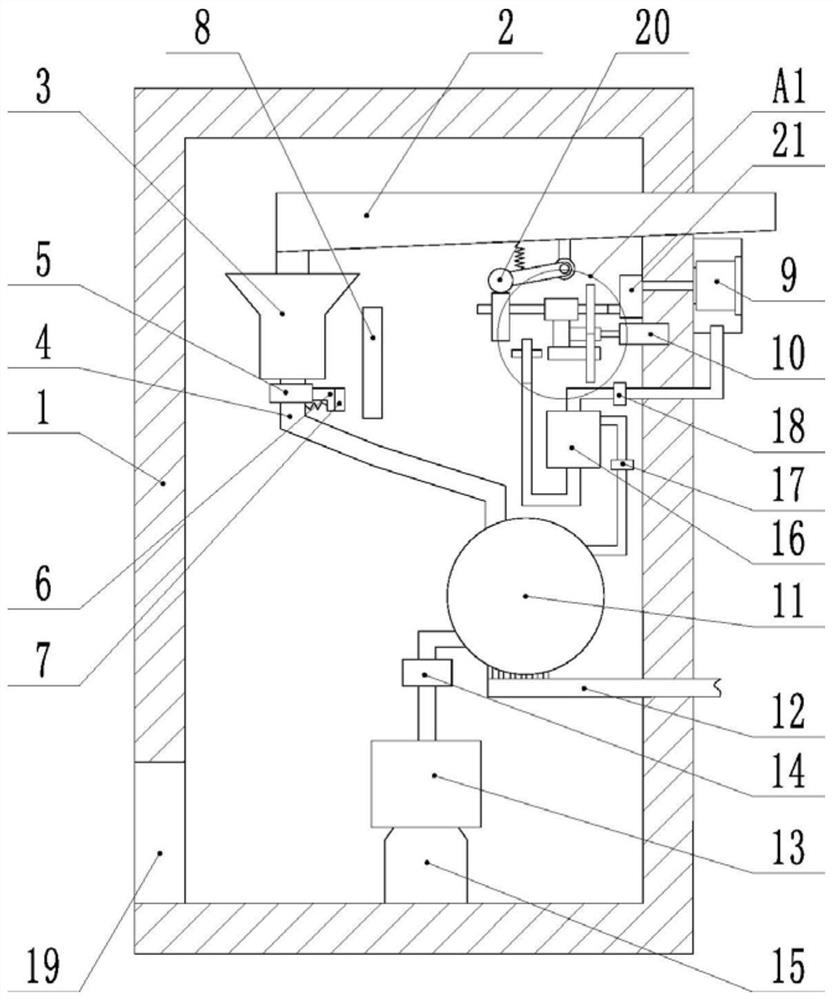
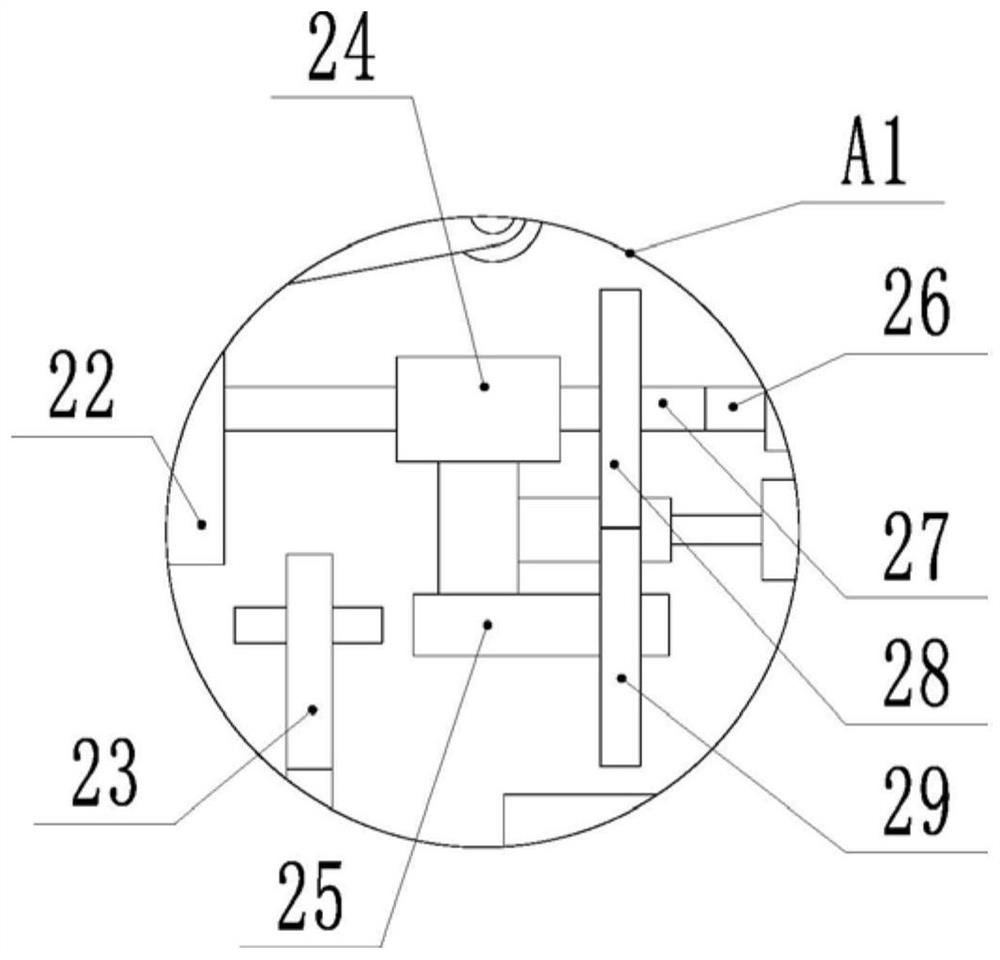
[0026] Embodiment: the aseptic refinement process of the original drug of pharmaceuticals, comprising the following steps:
[0027] A. Crude drug dissolution: put 100kg of crude drug into a dissolving tank filled with 400L of water for dissolution; keep the liquid temperature at 25°C, and stir with a stirring blade at a stirring speed of 90rpm. After dissolving, adjust pH with hydrochloric acid, add 4.5 to 5.5 kg of activated carbon, decolorize for 10 minutes, and then cool to 15°C.
[0028] B. Crystallization: The crystallization tank was first replaced with nitrogen 5 times, so that the crude drug solution passed through the activated carbon filter, the first filter, the second filter, and the filter plate of the first filter at a specified filtration pressure. The size is smaller than the size of the filter plate for activated carbon filtration, the size of the second filter plate is smaller than the size of the first filter plate, and transferred to the crystallization tan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com