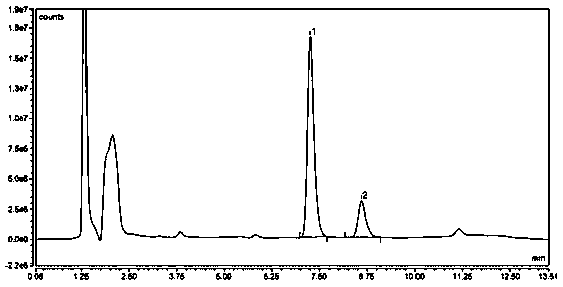
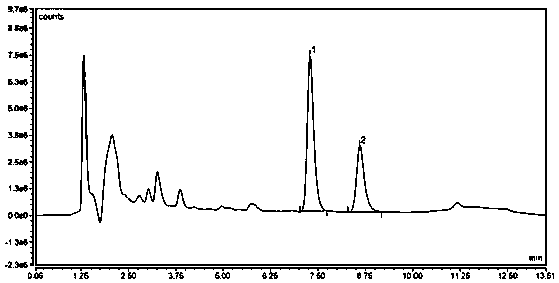
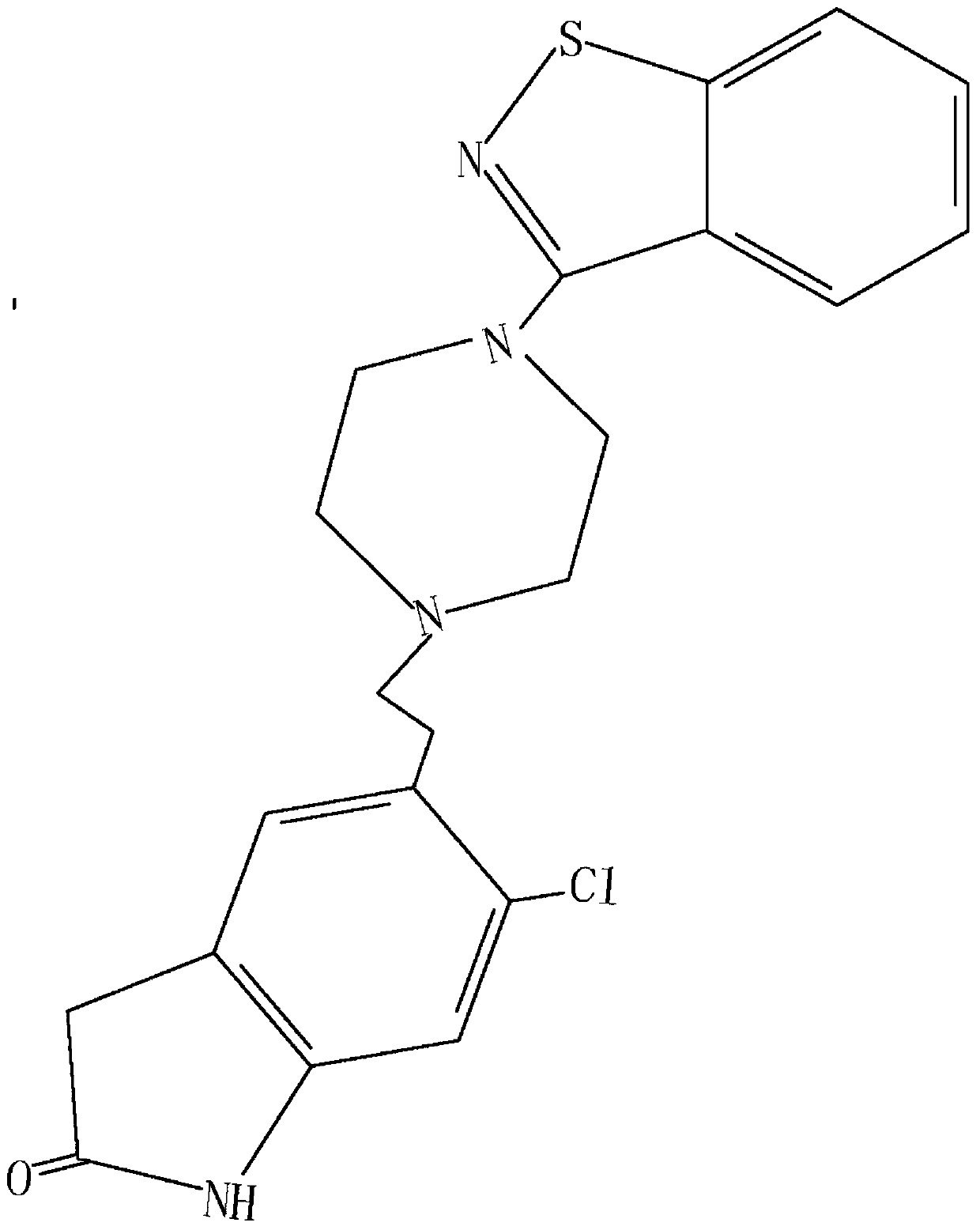
Liquid chromatographic analysis method for detecting ziprasidone content in blood
A liquid chromatography analysis and ziprasidone technology, which is applied in the field of detecting the concentration of the antipsychotic drug ziprasidone in the blood, can solve the problems of complex ziprasidone drug concentration processing and poor method specificity, and reduce toxicity and side effects Response, improved accuracy, and reduced experimental cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] A liquid chromatography analysis method for detecting ziprasidone content in blood of the present invention, it comprises the following steps:
[0037] (1) Calibration of standard solution
[0038] (a) Preparation of standard working solution:
[0039]Accurately weigh 10mg of ziprasidone standard substance and place it in a 10mL volumetric flask, dissolve it with 10mL of methanol to obtain standard stock solution A, the concentration of its ziprasidone is 1000ug / mL, and use standard stock solution A with a water content of Dilute with 50% methanol solution, use ziprasidone solutions containing 300, 400, 800, 1600, 3200, 6400 and 12800ng / mL respectively as the standard working solution, and store at -80°C;
[0040] (b) Preparation of internal standard working solution
[0041] Accurately weigh 10 mg of verapamil standard substance in a 10 mL test tube, dissolve with 10 mL of methanol to obtain internal standard stock solution B, whose verapamil concentration is 1000 ug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com