Calixarene derivative stationary phase, capillary gas chromatography column and preparation and application of calixarene derivative stationary phase
A gas chromatography column and derivative technology, which is applied in the field of capillary gas chromatography column and calixarene derivative stationary phase, can solve the problems of poor solubility and low column efficiency, and achieves low production cost, simple operation process and rich functional groups. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
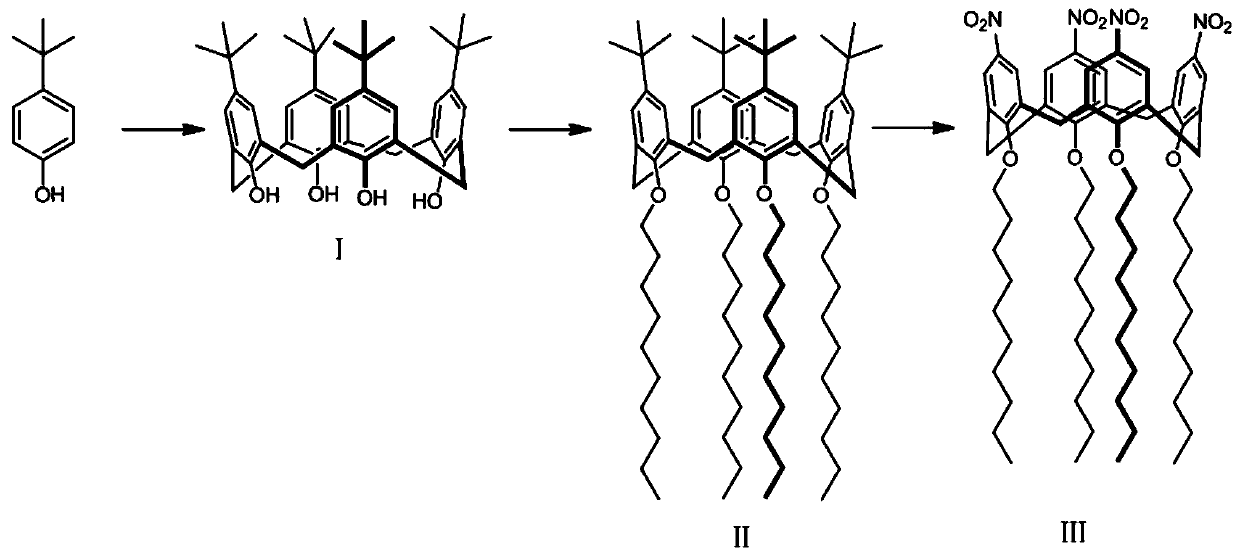
[0061] Add 10.02g (66.57mmol) of p-tert-butylphenol, 6.2mL (67.30mmol) of 40% formaldehyde solution, and 0.13g (3.13mmol) of NaOH into the round-bottomed flask, then gradually raise the temperature to 115°C, stir and reflux, The reaction solution gradually became turbid and viscous, and a yellow viscous solid appeared after 4 hours of reaction. After adding 50 mL of diphenyl ether, the solid gradually dissolved, and foam was generated and gradually increased. When a large amount of foam is generated, nitrogen is passed into the solution for purging, and after 3 hours, the nitrogen purging is stopped after there are no bubbles in the reaction solution, and the reaction is continued for 2 hours. Add 70mL of ethyl acetate and continue to reflux for 1.5h. Off-white solids are produced. Remove the flask from the oil bath, place it in a cold trap at 0°C, and vacuum filter to obtain a white solid. The filter cake was rinsed with deionized water and dried to obtain 2.42 g ...
Embodiment 2
[0066] Embodiment 2: the difference between this embodiment and embodiment 1 is:
[0067] Add 20.01g (133.22mmol) of p-tert-butylphenol, 12.40mL (134.61mmol) of 40% formaldehyde solution, and 0.25g (6.13mmol) of NaOH into the round-bottomed flask, and gradually raise the temperature from 90°C to 115°C, and stir. Reflux, the reaction solution gradually becomes turbid and viscous, and a yellow viscous solid appears after 4.5 hours of reaction. Add 100mL of diphenyl ether, the solid gradually dissolves, and foams gradually increase. After there were no bubbles in the reaction solution, the nitrogen purging was stopped, and the reaction was continued for 3 h. Add 140mL of ethyl acetate and continue to reflux for 1h. A yellow solid is produced. The flask is removed from the oil bath, placed in a cold trap at 0°C, and vacuum-filtered to obtain a light yellow solid. The filter cake was rinsed with deionized water and dried to obtain 8.22 g of intermediate (I) as a white solid powder...
Embodiment 3
[0070] Embodiment 3: the difference between this embodiment and embodiment 1 is:
[0071]Add 20.08g (133.65mmol) of p-tert-butylphenol, 12.4mL (134.61mmol) of 40% formaldehyde solution, and 0.25g (6.14mmol) of NaOH into the round-bottomed flask, and gradually raise the temperature from 94°C to 115°C, and stir. Reflux, the reaction solution gradually becomes turbid and viscous, and a yellow viscous solid appears after stirring for 4 hours. Add 100mL of diphenyl ether, the solid gradually dissolves, and foams are generated and gradually increase. After there were no bubbles in the reaction solution, the nitrogen purging was stopped, and the reaction was continued for 1 h. Add 140 mL of ethyl acetate and continue to reflux for 2 hours. A large amount of white solids are produced. The flask is removed from the oil bath, placed in a cold trap at 0°C, and vacuum filtered to obtain a light yellow solid. The filter cake was rinsed with 10 mL of deionized water, and dried to obtain 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com