Application of atisane type diterpenoid
A technology of attane and diterpenes, applied in the field of traditional Chinese medicine, can solve the problems such as angiogenesis that have not been reported for the time being
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 compound extraction, separation
[0023] 1. Extraction and separation
[0024] Dry and pulverize 2.0kg stems and leaves of King Kong, and soak 3 times with 95% ethanol, each time for 7 days, the extracts are combined, concentrated under reduced pressure to obtain extract (200g), and extracted with ethyl acetate to obtain the ethyl acetate part ( 80g). Use MCI-gel (30%-100%) for separation, wherein the second part is separated with silica gel, the eluent is petroleum ether-acetone (20:1-1:1), select the third part, and use reverse phase liquid Separated by phase chromatography, eluted with acetonitrile / water (50:50, 3mL / min), and collected for 13 minutes to obtain the compound.
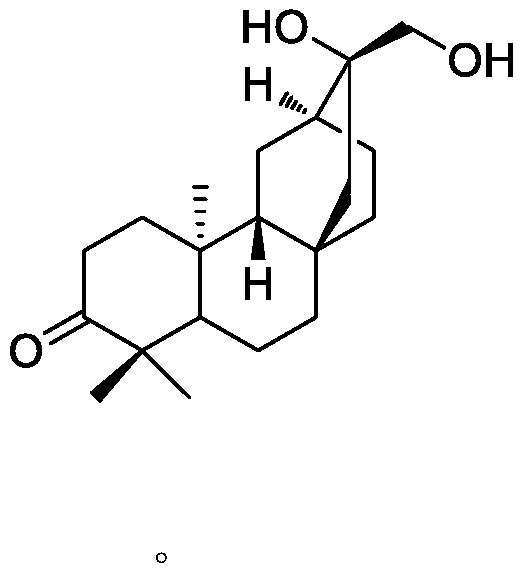
[0025] 2. Identification of the structure of the compound
[0026] The compound is a white powder, 1 H NMR (400MHz, CDCl 3 ,δin ppm,J in Hz):δ H 1.07(3H,s,H-18),1.04(3H,s,H-19),1.10(3H,s,H-20),3.56(1H,d,J=8.1Hz,H-17a),3.42 (1H, d, J = 8.1 Hz, H-17b). 13 C NMR (125MHz, CDCl 3 ...
Embodiment 2
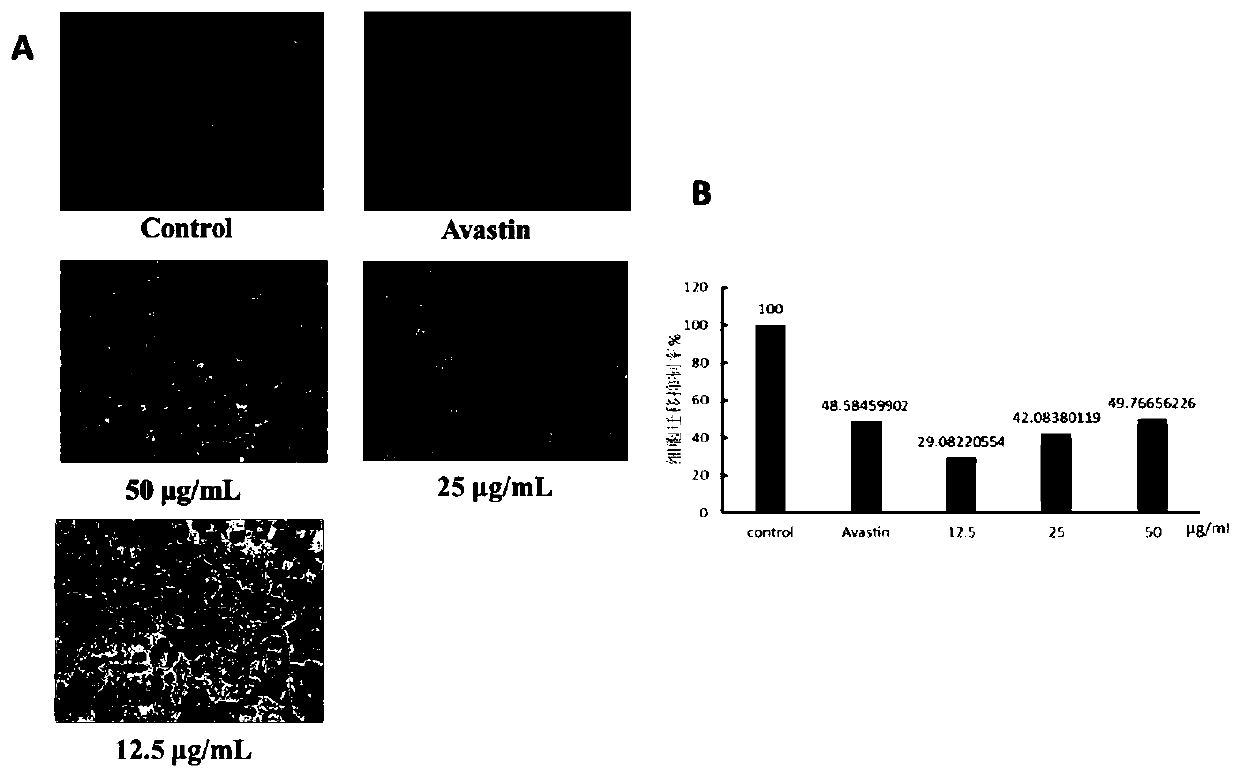
[0027] Inhibition of endothelial cell migration by compound of embodiment 2 1. Experimental materials, instruments and reagents
[0028] 1 Experimental materials
[0029] 1.1 Drugs: Compounds (atisane-3-oxo-16,17-diol) isolated in our laboratory
[0030] Avastin
[0031] 1.2 Cell lines:
[0032] Human umbilical vein endothelial cells (HUVEC) were preserved by our research group.
[0033] 2 Preparation methods of main test reagents
[0034] 2.1 DMEM preparation method: take 10.4g DMEM dry powder, 2g NaHCO 3 , 50mg penicillin, 100mg streptomycin, ddH 2 O 800mL, fully mixed with a magnetic stirrer, adjusted to pH 7.4 with HCl, sterilized by filtration with a 0.22μm filter, aliquoted, and stored at 4°C for later use.
[0035] 2.2 PBS liquid preparation method: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.24g, add water to make up to 1L, sterilize under high temperature and high pressure, and store at 4°C for later use.
[0036] 2.3 Preparation method of trypsi...
Embodiment 3
[0052] Inhibition of Embodiment 3 Compounds Forming Rat Arterial Rings 1. Experimental Materials, Instruments and Reagents
[0053] 1 Experimental materials
[0054] 1.1 Drugs: Compounds (atisane-3-oxo-16,17-diol) isolated in our laboratory
[0055] 1.2 Animals:
[0056] SD rats, SPF grade, female, body weight (250) g
[0057] 2 Preparation methods of main test reagents
[0058] 2.1 DMEM preparation method: take 10.4g DMEM dry powder, 2g NaHCO 3 , 50mg penicillin, 100mg streptomycin, ddH 2 O 800mL, fully mixed with a magnetic stirrer, adjusted to pH 7.4 with HCl, sterilized by filtration with a 0.22μm filter, aliquoted, and stored at 4°C for later use.
[0059] 2.2 PBS liquid preparation method: NaCl 8.0g, KCl 0.2g, NaCl 2 HPO 4 1.44g, KH 2 PO 4 0.24g, add water to make up to 1L, sterilize under high temperature and high pressure, and store at 4°C for later use.
[0060] 2.3 Matrigel solution: Dilute Matrigel 1:1 with serum-free ECM medium.
[0061] 2. Experimenta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com