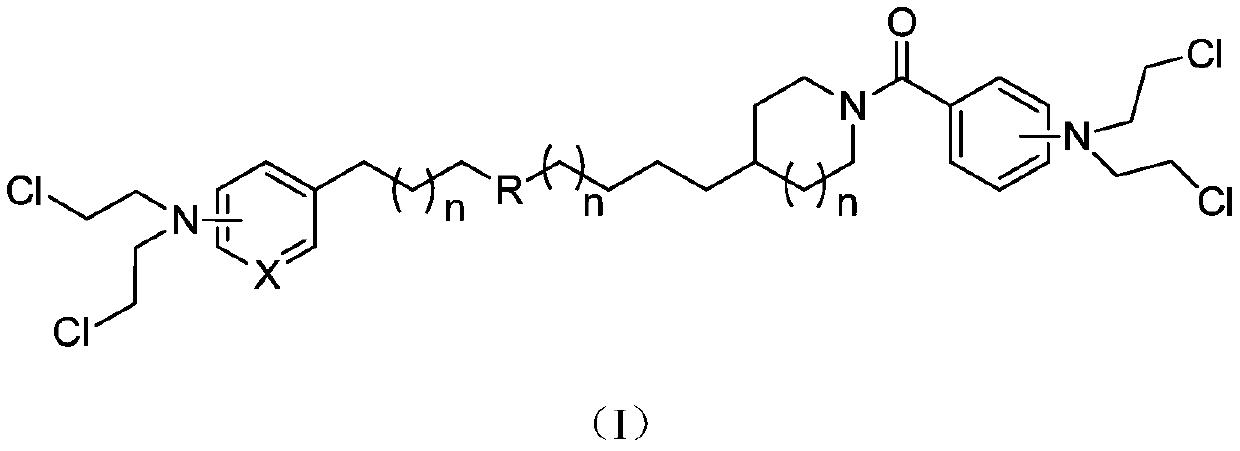
Multi-target nicotinamide phosphoribosyl transferase nitrogen mustard inhibitor with antitumor activity and preparation and application thereof
A technology of anti-tumor activity and phosphoribose, which is applied in the field of medicine to achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example one (E)-N-(4-(1-(4-(bis(2-chloroethyl)amino)benzoyl)piperidin-4-yl)butyl)-3-(pyridine-3 -Based) acrylamide preparation
[0066] (1) Preparation of intermediate 2c: methyl 4-(1-(4-nitrobenzoyl)piperidin-4-yl)butyrate
[0067] Dissolve compound 1 (1.0g, 4.8mmol) in methanol (30mL), and add SOCl dropwise 2 (1.1g, 9.6mmol), react for 4h. After the reaction, the solvent was evaporated under reduced pressure and dissolved in dichloromethane (30mL), and 4-nitrobenzoyl chloride (1.8g, 9.6mmol), NaHCO 3 (0.8g, 9.6mmol), react at room temperature overnight. After the reaction, the solvent was evaporated under reduced pressure. The residue was purified by column chromatography using a dichloromethane / methanol mixed solvent (100:1) as the mobile phase to obtain Intermediate 2c as a colorless oil, 0.9 g in total, and 57% yield.
[0068] 1 H-NMR(DMSO-d 6 ,300MHz)δ:7.25(d,J=8.64Hz,2H), 6.75(d,J=8.64Hz,2H), 3.56(s,3H), 2.74(t,J=7.54Hz,2H), 2.26( t,J=7.54Hz,2H),1.78-1.89(m,3H),1.62...
Embodiment 2
[0085] Example 2 Compound 7a~c, 9a~c, 12 and 14 enzyme inhibitory activity and in vitro antitumor activity
[0086] 1 Compound 7a~c, 9a~c, 12 and 14 inhibit NAMPT enzyme
[0087] The enzyme described below refers specifically to NAMPT.
[0088] 1.1 Preparation of NAMPT enzyme
[0089] BL21(DE3)plysS cells transformed with the recombinant plasmid (NAMPT-pET28a+) were inoculated into 2×YT medium (37 μg / mL chloramphenicol and 100 μg / mL kanamycin). The cells are collected by induction and centrifugation, and the cells are lysed. The supernatant is incubated with the Ni-NTA column (purchased from QIAGEN) on ice to wash away the impurities, and finally the target protein is eluted, and the protein is finally obtained.
[0090] 1.2 Experimental method
[0091] First add 0.5μL of compound solutions of different concentrations to a 96-well plate, then add 20μL of enzyme reaction mixture solution (enzyme reaction components other than the substrate), incubate at room temperature for 5 minutes, ad...
Embodiment 3
[0117] Example 3 Anti-tumor effect of target compound in vivo
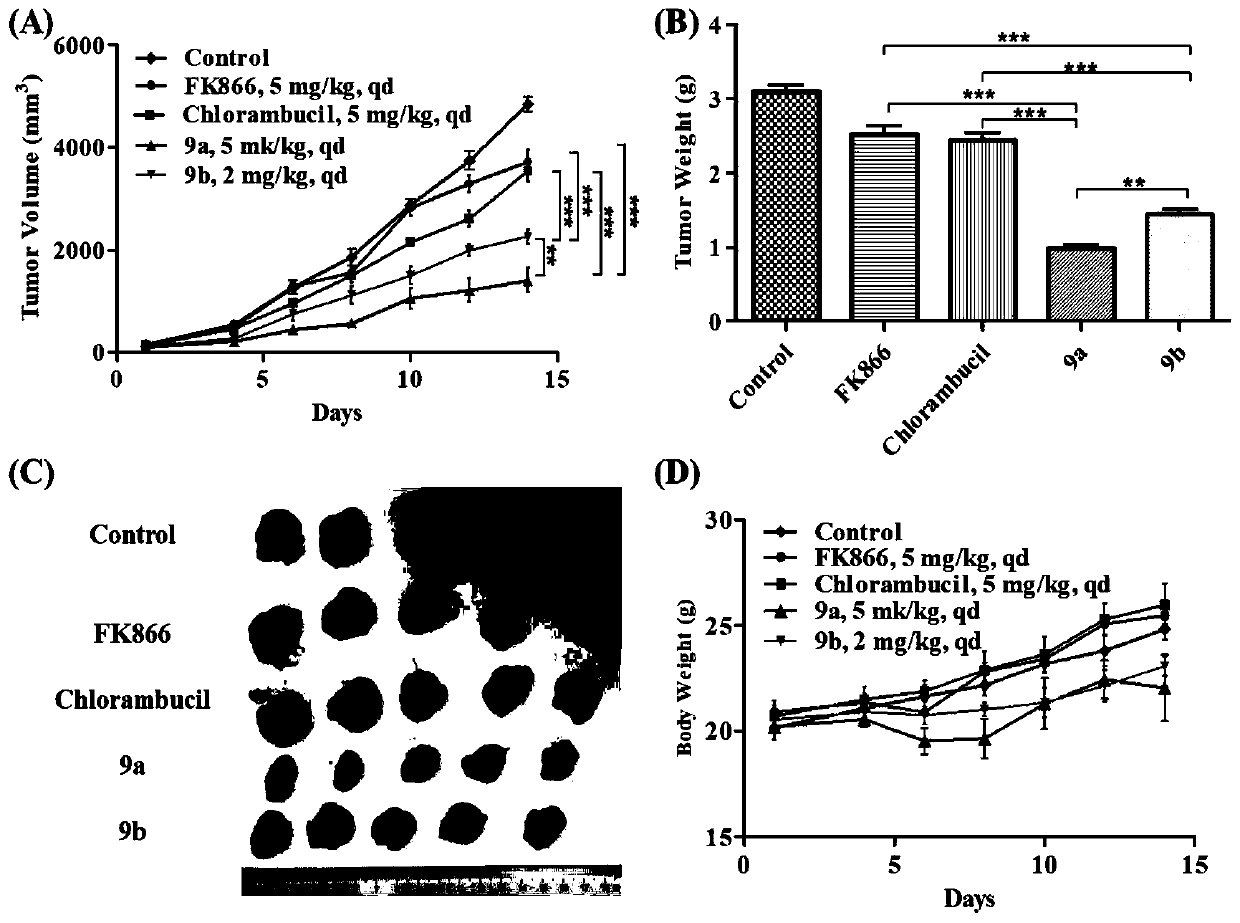
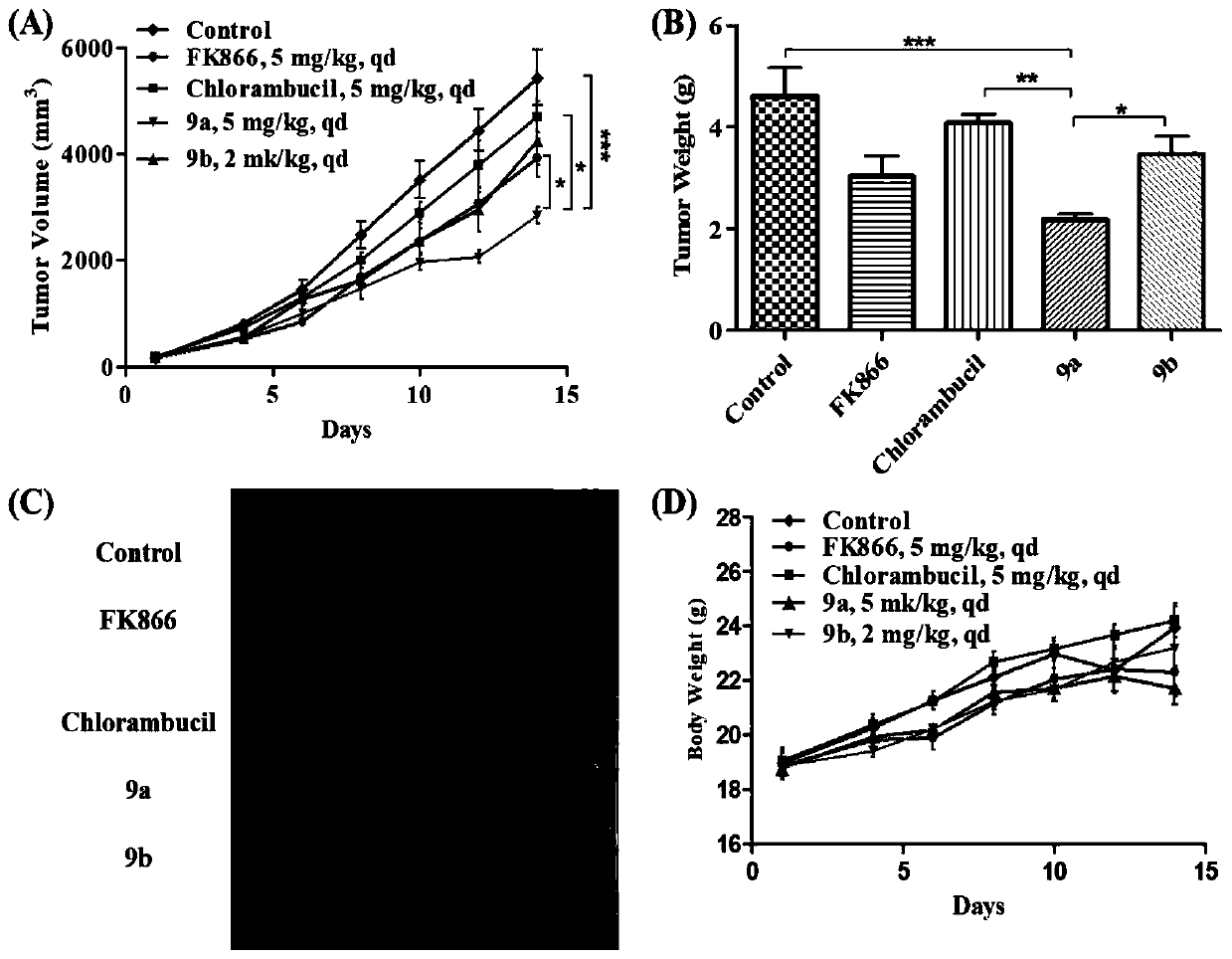
[0118] 1 The effect of compound 9a and 9b on the transplanted tumor of CT-26 normal mouse colon cancer
[0119] According to the results of in vitro anti-tumor activity, firstly, the in vivo anti-tumor activity of compounds 9a and 9b was evaluated with the mouse colon cancer CT-26 normal mouse xenograft model. FK866 and chlorambucil were the positive control drugs.
[0120] The dosage of compound 9a, FK866 and chlorambucil was 5 mg / kg, once a day; compound 9b was 2 mg / kg, once a day; continuous intraperitoneal injection was administered for 14 days. The results show that( figure 1 ), compounds 9a and 9b can effectively inhibit tumor growth, and the tumor inhibition rates are 71.1% and 53.5%, respectively, which are significantly higher than the positive control drug (FK866 tumor inhibition rate is 18.9%, chlorambucil is 21.6%), of which compound 9a is in vivo Anti-tumor activity is better than 9b, and all have statistic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com