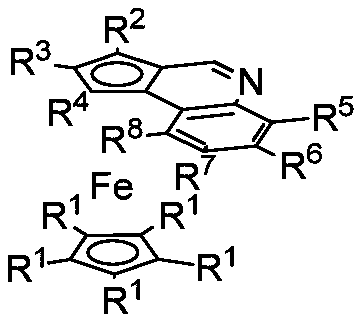
A class of ferrocenoquinoline compounds with face chirality and its synthesis method
A chiral ferrocene and compound technology, applied in chemical instruments and methods, organic chemistry, metallocene and other directions, to achieve the effect of less reaction steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
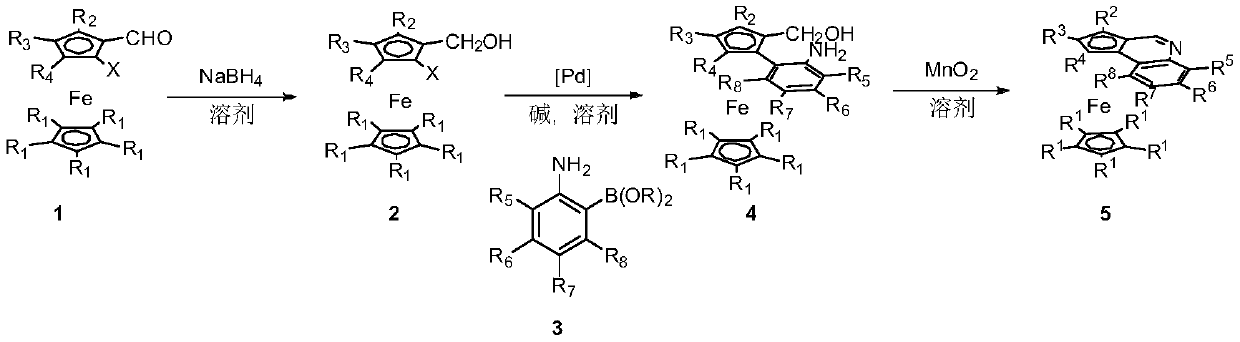
Examples
Embodiment 1
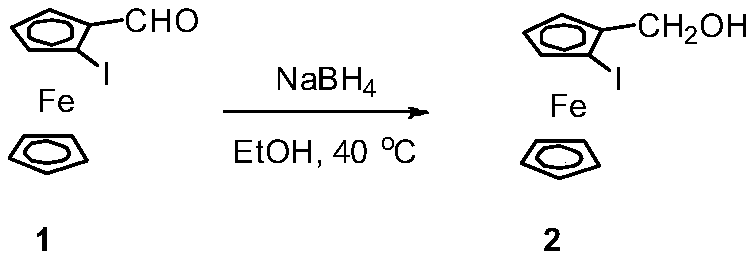
[0031] The synthesis of embodiment 1 compound 2
[0032]
[0033] Under nitrogen protection, add compound 1 (1.049g, 3.1mmol) and 10mL EtOH to a 100mL reaction flask, raise the temperature to 40°C, and slowly add NaBH 4 (176mg, 4.5mmol), stirred overnight. Add 10mL H 2 O quenches the reaction. Dichloromethane extraction, anhydrous Na 2 SO 4 After drying, column chromatography gave 0.960 g of a light yellow solid with a yield of 91%.
Embodiment 2
[0034] The synthesis of embodiment 2 compound 4a
[0035]
[0036] Under nitrogen protection, compound 2 (260mg, 0.76mmol), compound 3a (230mmg, 1.52mmol), Pd(OAc) were added to the 50mL reaction flask 2 (34mg, 0.152mmol), Ba(OH) 2 ·8H 2 O (480 mg, 1.52 mmol), 10 mL ethanol and 1 mL water. Raise the temperature to 100°C and react overnight. Cool to room temperature, add 20mL H 2 O, dichloromethane extraction, anhydrous Na 2 SO 4 Drying and column chromatography gave 0.170 g of a light yellow solid with a yield of 73%. 1 H NMR (400MHz, CDCl 3 )δ7.67(dd, J=7.6,1.5Hz,1H),7.18-7.12(m,1H),6.92-6.86(m,1H),6.72(dd,J=7.9,0.8Hz,1H),4.46 (t,J=6.5,5.1Hz,1H),4.30-4.27(m,3H),4.23(s,5H),3.76(s,2H); 13 C NMR (100MHz, CDCl 3 )δ144.6, 133.0, 128.3, 123.2, 119.5, 116.1, 87.7, 85.9, 71.0, 69.6, 69.5, 67.6, 59.9. HRMS: Calculated for C 17 h 17 56 FeNONa[M+Na] + 330.0557,found 330.0555.
Embodiment 3
[0037] The synthesis of embodiment 3 compound 4b
[0038]
[0039] Under nitrogen protection, compound 2 (260mg, 0.76mmol), compound 3b (254mmg, 1.52mmol), Pd(OAc) were added to the 50mL reaction flask 2 (34mg, 0.152mmol), Ba(OH) 2 ·8H 2 O (480 mg, 1.52 mmol), 10 mL ethanol and 1 mL water. Raise the temperature to 100°C and react overnight. Cool to room temperature, add 20mL H 2 O, dichloromethane extraction, anhydrous Na 2 SO 4 After drying, column chromatography gave 0.186 g of a light yellow solid with a yield of 73%. 1 H NMR (400MHz, CDCl 3 )δ7.57 (d, J = 8.4Hz, 1H), 6.46 (dd, J = 8.4, 2.4Hz, 1H), 6.28 (d, J = 2.4Hz, 1H), 4.46 (s, 1H), 4.31- 4.25(m,3H),4.25-4.19(m,6H),3.80(s,3H),3.64(brs,3H); 13 C NMR (100MHz, CDCl 3)δ160.0, 145.8, 133.8, 115.4, 104.9, 101.7, 87.5, 86.4, 70.8, 69.5, 67.5, 60.0, 55.3. HRMS Calculated for C 18 h 19 56 FeNO 2 Na[M+Na] + 360.0663,found 360.0656.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com