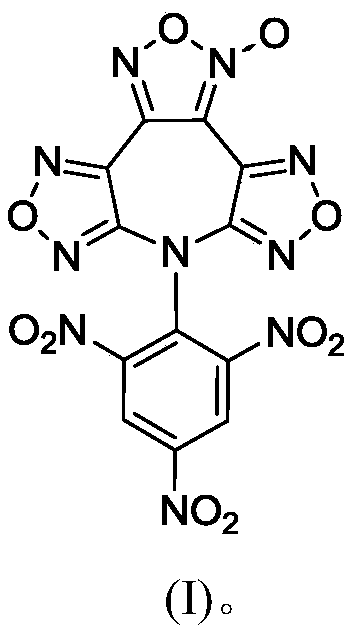
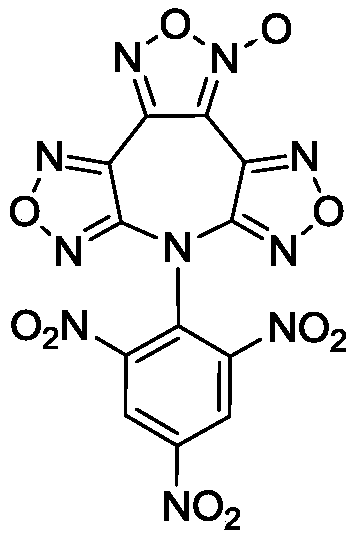
7-(2,4,6-Trinitrophenyl)difurazano-furoxano-azepine compound
A technology of trinitrophenyl and furazan oxide is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, organic chemistry, etc., and can solve the problems of low density, low thermal decomposition temperature, low detonation speed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At a temperature of 25°C, 7H-bisfurazolo[3,4-b:3',4'-f]furazolo[3",4"-d]azepine (0.47g , 2mmol) and 1-chloro-2,4,6-trinitrobenzene (0.495g, 2mmol) were added into anhydrous acetonitrile (5mL), stirred and dissolved. Slowly add NEt dropwise to it 3 (0.404g, 4mmol), continue to stir the reaction for 4h. After the reaction was completed, the solvent was evaporated to dryness, and the resulting crude product was rinsed with absolute ethanol to obtain brown powder 7-(2,4,6-trinitrophenyl)bisfurazanofurazanazepine 0.70g, yield 78.5%, purity 98.6%.
[0020] Structure Identification:
[0021] Infrared Spectrum: IR(KBr,cm -1 )ν: 3091, 1653, 1617, 1544, 1474, 1339, 1155, 1089, 996, 975, 920, 707;
[0022] NMR spectrum:
[0023] 1 H NMR (acetone-d 6 ,500MHz,ppm),δ:9.50(s,2H,C 6 h 2 );
[0024] 13 C NMR (acetone-d 6 ,125MHz,ppm),δ:153.62,153.07,150.42,148.53,144.17,137.70,135.53,128.96,127.18,105.51;
[0025] Elemental Analysis: Structural Formula C 12 h 2 N 10 o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com