Pyrazolone pyrimidine compound as well as preparation method and application thereof
A technology of pyrazolone and pyrimidine, which is applied in the field of pyrazolone and pyrimidine compounds, and can solve the problems of poor inhibitory activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0512]
[0513] first step:
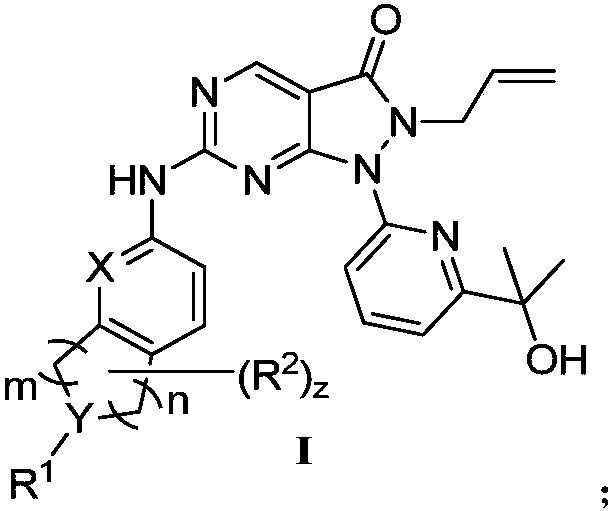
[0514] 2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-6-methylthiopyrazolo[3,4-d]pyrimidine-3 -Ketone (270mg, 0.75mmol) (compound as shown in formula 1A) and 4-chloroperoxybenzoic acid (143mg, 0.83mmol) were dissolved in toluene (20ml), stirred at room temperature for 1 hour, and then added N,N- Diisopropylethylamine (291mg, 2.26mmol) and 6-amino-3,4-dihydro-1H-isoquinoline-2-carboxylic acid tert-butyl ester (243mg, 0.98mmol) (as shown in formula 2 Compound), continue to stir at room temperature for 16 hours, the reaction solution was concentrated, purified by silica gel column chromatography (petroleum ether / ethyl acetate = 100% to 50%) to obtain the compound represented by formula I-1-a tert-butyl 6-[ [2-Allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-3-oxo-pyrazolo[3,4-d]pyrimidine-6 -Yl]amino]-3,4-dihydro-1H-isoquinoline-2-carboxylic acid ethyl ester (270 mg, 0.48 mmol). LC-MS:m / z:(M+H) + = 558.3, 1 H NMR(400MHz, CDCl 3 )δ8.88(s,1H),7.91(...
Embodiment 2
[0518]
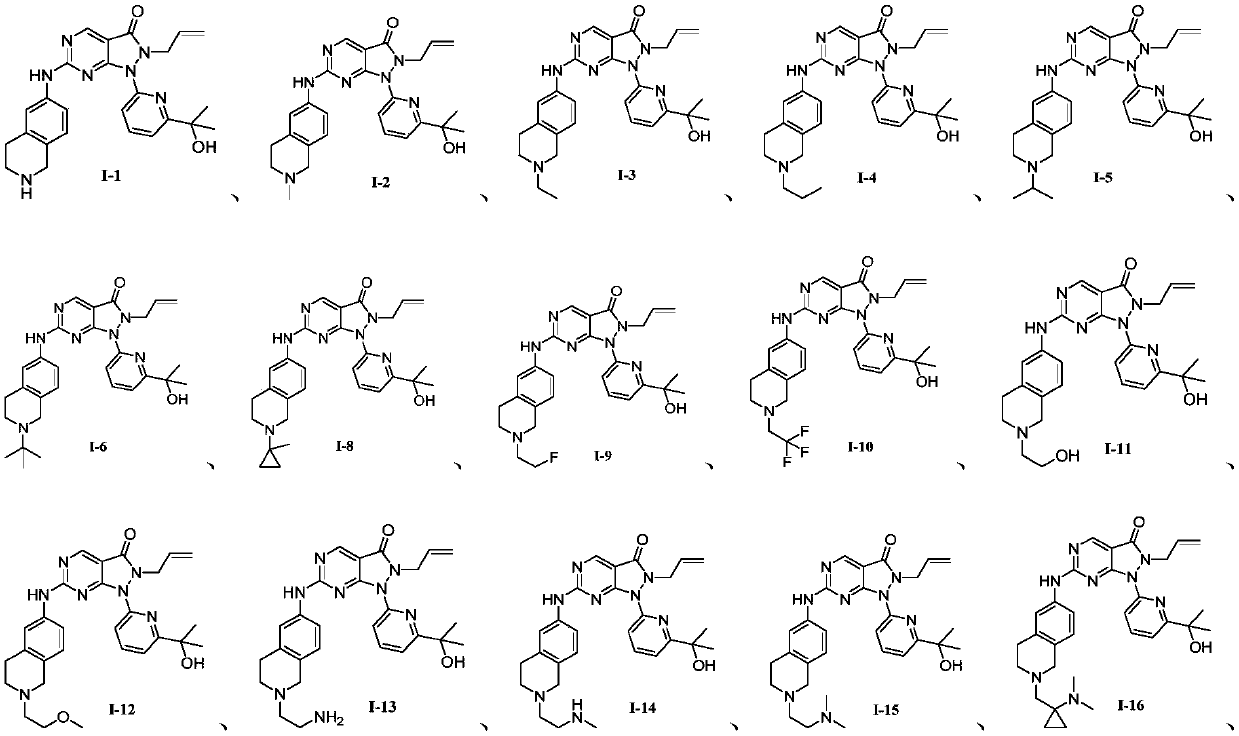
[0519] The 2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-6-(1,2,3,4-tetrahydroisoquinoline-6 -Ylamino)pyrazolo[3,4-D]pyrimidin-3-one (40mg, 0.087mmol) (compound as shown in formula I-1) was dissolved in methanol (5ml), and then an aqueous formaldehyde solution ( 0.1ml, 40%) and sodium triacetylborohydride (46mg, 0.22mmol) were stirred at room temperature for 4 hours. The reaction solution was concentrated, dissolved with a mixture of dichloromethane and methanol (10 / 1, 20ml), washed twice with saturated sodium bicarbonate, the organic layer was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain the formula I-2 The compound shown is 2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-6-[(2-methyl-3,4-dihydro- 1H-Isoquinolin-6-yl)amino]pyrazolo[3,4-d]pyrimidin-3-one (40 mg, 0.085 mmol). LC-MS:m / z:(M+H) + =472.3, 1 H NMR(400MHz, DMSO-d 6 )δ10.23(s,1H),8.89(s,1H),8.05(t,J=7.9Hz,1H),7.80(d,J=8.0Hz,1H),7.66(m,2H),7.40( d,J=7.6Hz,1H), 7.01(d,J=8....
Embodiment 5
[0521]
[0522] The 2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-6-(1,2,3,4-tetrahydroisoquinoline-6 -Amino)pyrazolo[3,4-D]pyrimidin-3-one (40mg, 0.087mmol) (compound as shown in formula I-1) was dissolved in dichloromethane (5ml), and then acetone ( 0.1ml, 40%) and sodium triacetylborohydride (46mg, 0.22mmol), heated to reflux and stirred for 48 hours. The reaction solution was concentrated and purified by silica gel column chromatography (dichloromethane / methanol=100% to 10%, then dichloromethane / methanol / ammonia methanol solution=100 / 10 / 1.5) to obtain the compound represented by formula I-5 2-allyl-1-[6-(1-hydroxy-1-methyl-ethyl)-2-pyridyl]-6-[(2-isopropyl-3,4-dihydro-1H- Isoquinolin-6-yl)amino]pyrazolo[3,4-d]pyrimidin-3-one (30 mg, 0.06 mmol). LC-MS:m / z:(M+H) + =496.2, 1 H NMR(400MHz, MeOD-d 4 )δ8.87(s,1H), 8.05(t,J=7.8Hz,1H),7.86-7.76(m,2H),7.70(d,J=7.8Hz,1H),7.51(d,J=7.6 Hz, 1H), 7.18 (d, J = 8.5 Hz, 1H), 5.74 (dq, J = 11.0, 6.2 Hz, 1H), 5.06 (d, J = 10.1 Hz, 1H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com