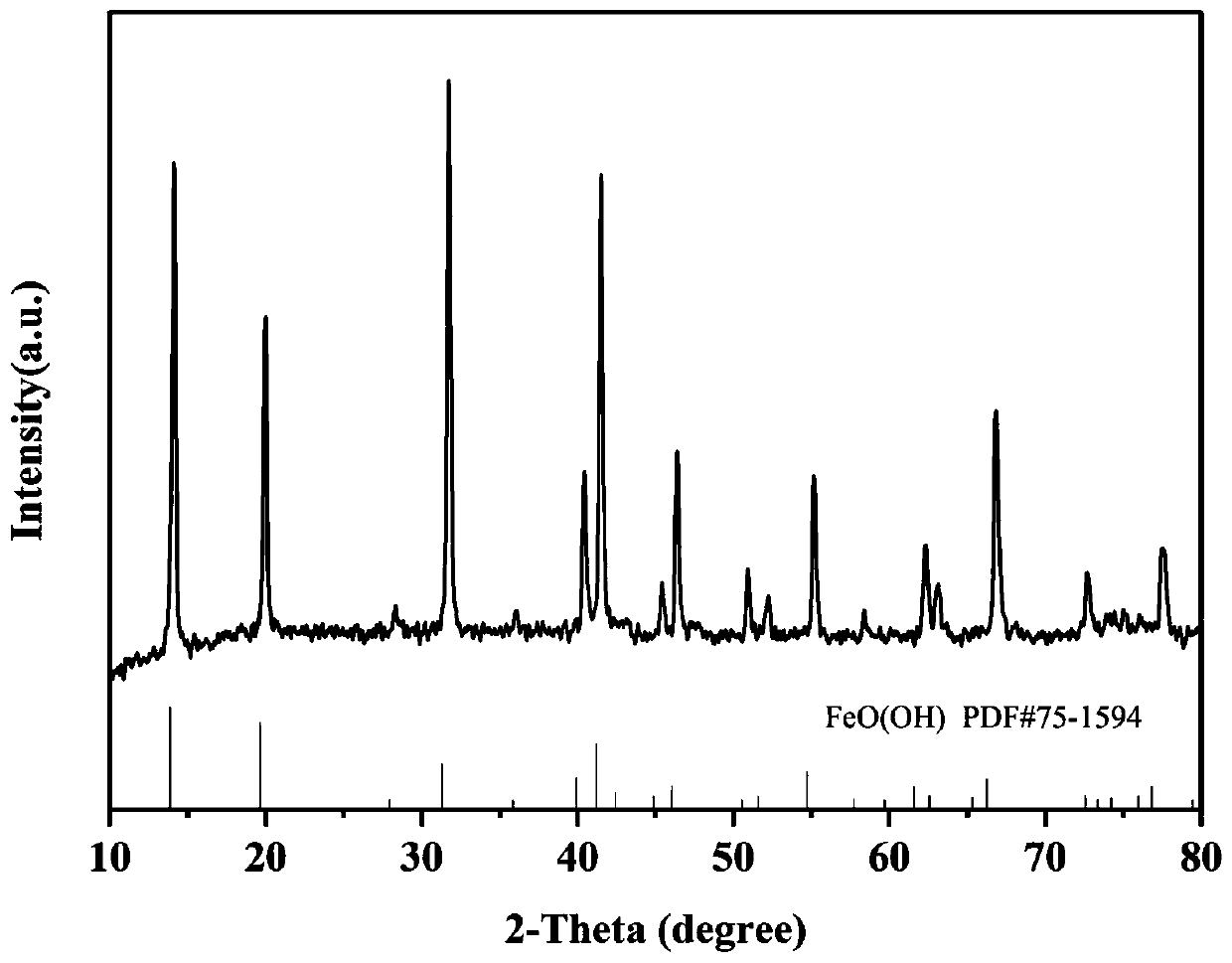
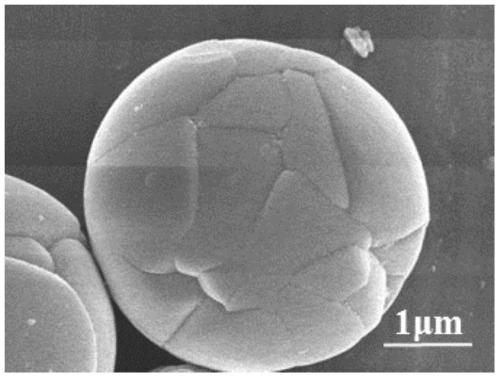
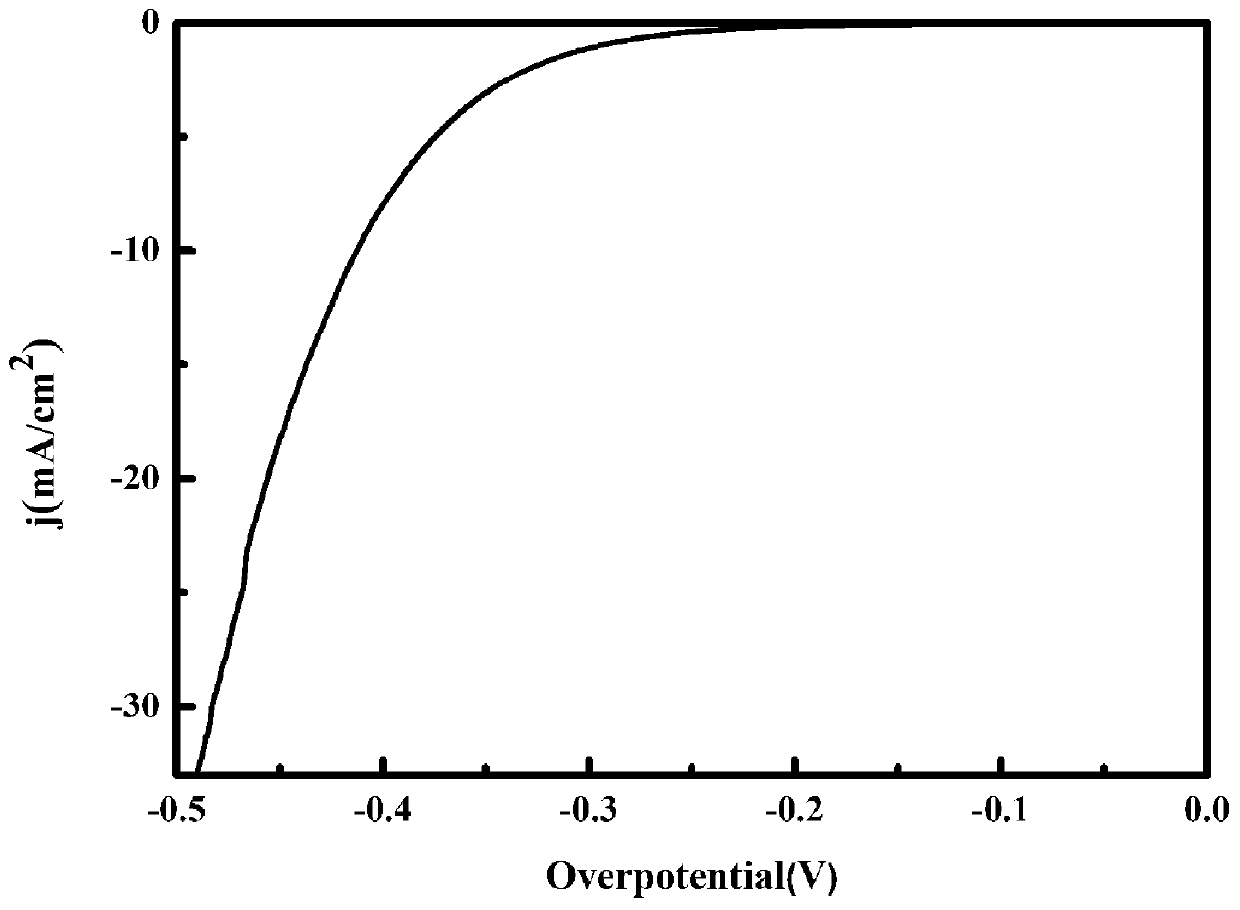
Preparation method of vanadium-doped iron oxyhydroxideelectrocatalyst
An iron oxyhydroxide, electrocatalyst technology, applied in catalyst activation/preparation, physical/chemical process catalyst, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problem that elemental iron is easy to corrode iron oxides and reduce activity , HER overpotential problems, to achieve the effect of enhanced electrochemical performance, short cycle, low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Take FeCl 3 ·6H 2 O, VCl 3 , CO(NH 2 ) 2 and NH 4 F was added to 10ml deionized water at the same time, and magnetically stirred at room temperature for 10 minutes to obtain a concentration of iron source of 0.08mol / L, a concentration of vanadium source of 0.035mol / L, CO(NH 2 ) 2 The concentration is 0.5mol / L, NH 4 The concentration of F is a clear solution A of 0.3mol / L;
[0022] 2) Pour the clarified solution A into the reaction liner at a filling ratio of 40% and seal it, then install the liner in the outer kettle and fix it, place it in a homogeneous reactor, and then react at 90°C for 10 hours;
[0023] 3) After the hydrothermal reaction is over, cool the reaction kettle to room temperature naturally, take out the reaction solution, and collect the product through alternating centrifugal washing with water and absolute ethanol for 3 times. After vacuum drying at 50°C, pour it into a mortar and grind it into fine particles. Powdered samples, that is, nano...
Embodiment 2
[0025] 1) Take FeCl 3 ·6H 2 O, VCl 3 , CO(NH 2 ) 2 and NH 4 F was added to 20ml of deionized water at the same time, and magnetically stirred at room temperature for 20 minutes to obtain a concentration of iron source of 0.045mol / L, a concentration of vanadium source of 0.0125mol / L, CO(NH 2 ) 2 The concentration is 0.5mol / L, NH 4 The concentration of F is a clear solution A of 0.2mol / L;
[0026] 2) Pour the clarified solution A into the reaction liner at a filling ratio of 60% and seal it, then install the liner in the outer kettle and fix it, place it in a homogeneous reactor, and react at 110°C for 15 hours;
[0027] 3) After the hydrothermal reaction is over, cool the reaction kettle to room temperature naturally, take out the reaction solution, and collect the product through alternating centrifugal washing with water and absolute ethanol for 3 times. After vacuum drying at 60°C, pour it into a mortar and grind it into fine particles. Powdered samples, that is, nan...
Embodiment 3
[0029] 1) Take FeCl 3 ·6H 2 O, VCl 3 , CO(NH 2 ) 2 and NH 4 F was added to 30ml deionized water at the same time, and magnetically stirred at room temperature for 30min to obtain a concentration of iron source of 0.033mol / L, a concentration of vanadium source of 0.005mol / L, CO(NH 2 ) 2 The concentration is 0.5mol / L, NH 4 The concentration of F is the clear solution A of 0.167mol / L;
[0030] 2) Pour the clarified solution A into the reaction liner at a filling ratio of 80% and seal it, then install the liner in the outer kettle and fix it, place it in a homogeneous reactor, and react at 130°C for 20 hours;
[0031] 3) After the hydrothermal reaction is over, cool the reaction kettle to room temperature naturally, take out the reaction solution, and collect the product through alternate centrifugal washing with water and absolute ethanol for 3 times, dry it in vacuum at 70°C, pour it into a mortar, and grind it into fine particles. Powdered samples, that is, spherical va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com