boc-(r)-3-amino-4-(2,4,5-trifluorophenyl)butanoic acid condensation impurity and preparation method thereof
A technology of trifluorophenyl and amino groups, applied in the field of organic synthesis medicinal chemistry, can solve the problems of unfavorable industrial production, high cost, low general yield and the like, and achieve the effects of easy control, moderate reaction conditions, simple and efficient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
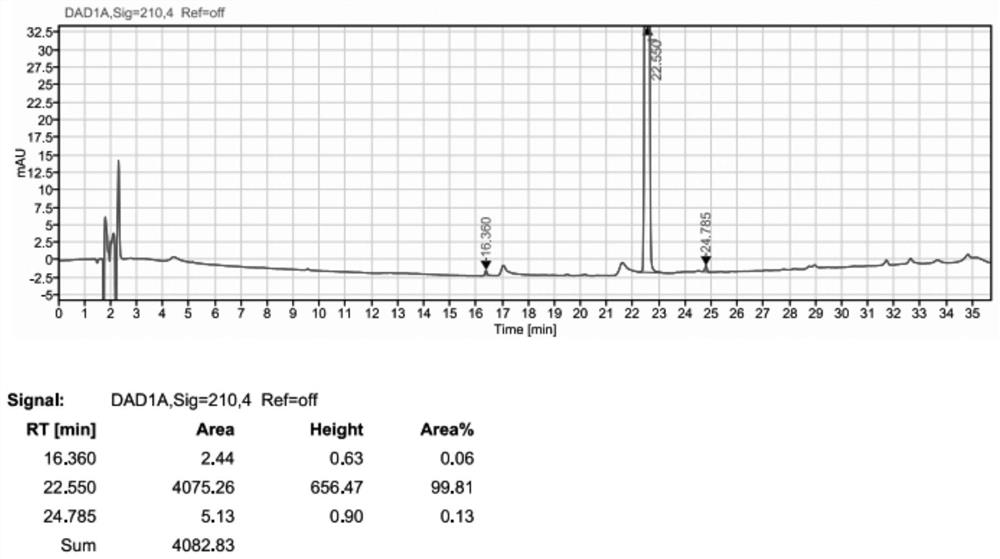
[0087] Example 1 Preparation of BOC- (R) -3-amino-4- (2,4,5-trifluorophenyl) butyric acid condensation impurities
[0088] 500 ml of four-mouth flask was input to 10 g (0.03 mol), (0.03 mol), (R) -3-amino-4- (2) (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) 4,5-trifluorophenyl) butyric acid 8 g (0.0297 mol), HOBT 5g (0.036 mol), EDC-HCl 7g (0.036 mol), and 90 g of acetonitrile, the solid was dispersed uniform. Triethylamine 4g (0.04 mol) was added dropwise, and the temperature was controlled from 20 to 30 ° C, and the insulation reaction was controlled until the end point. The acetonitrile was recovered under reduced pressure to give a solid crude material. The concentrate was purified by silica gel column (dichloromethane: ethyl acetate = 8: 1) Collecting eluent containing impurities, concentrated to dryness to give a white solid 11.5 g, purity of about 99%, yield of about 70%.
[0089] The solid obtained by preparing in Example 1 was analyze...
Embodiment 2
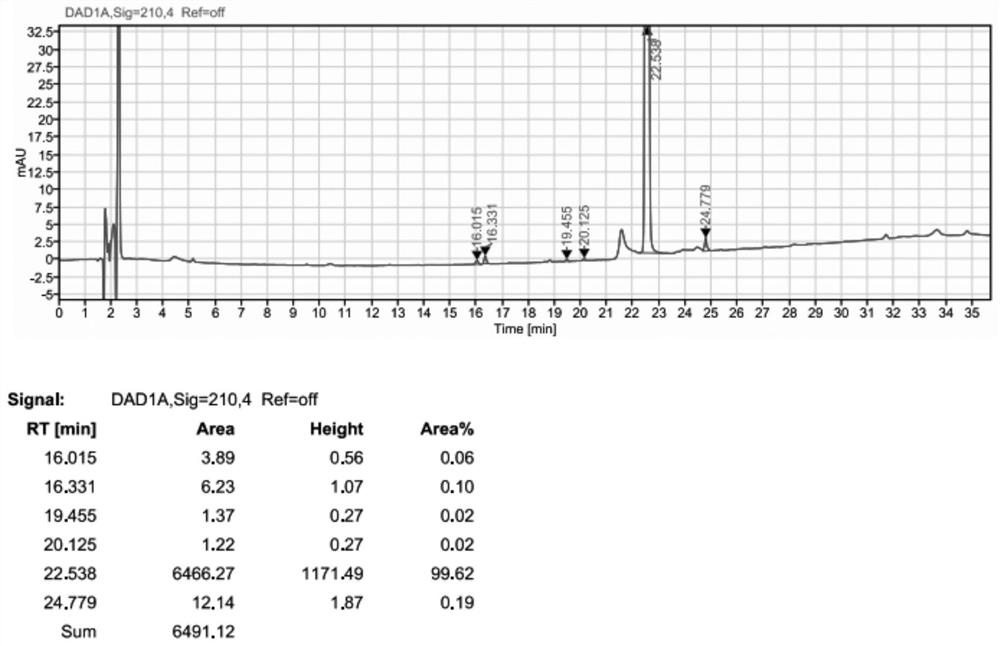
[0097] Example 2 Preparation of BOC- (R) -3-amino-4- (2,4,5-trifluorophenyl) butyric acid condensation impurities
[0098] 500 ml of four-mouth flask was input to 10 g (0.03 mol), (0.03 mol), (R) -3-amino-4- (2) (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) 4,5-trifluorophenyl) butyric acid 8 g (0.0297 mol), HOBT 5g (0.036 mol), EDC-HCl 7g (0.036 mol), and 90 g of acetonitrile, the solid was dispersed uniform. 2.4 g (0.04 mol) was added dropwise, and the temperature was controlled from 20 to 30 ° C, and the insulation reaction was controlled until the end point. The acetonitrile was recovered under reduced pressure to give a solid crude material. The concentrate was purified by silica gel column (dichloromethane: ethyl acetate = 8: 1) Collect eluent containing impurities, concentrated to dryness to give a white solid. The solid was 10.68 g, which was about 99% purity, and the yield was about 65%.
Embodiment 3
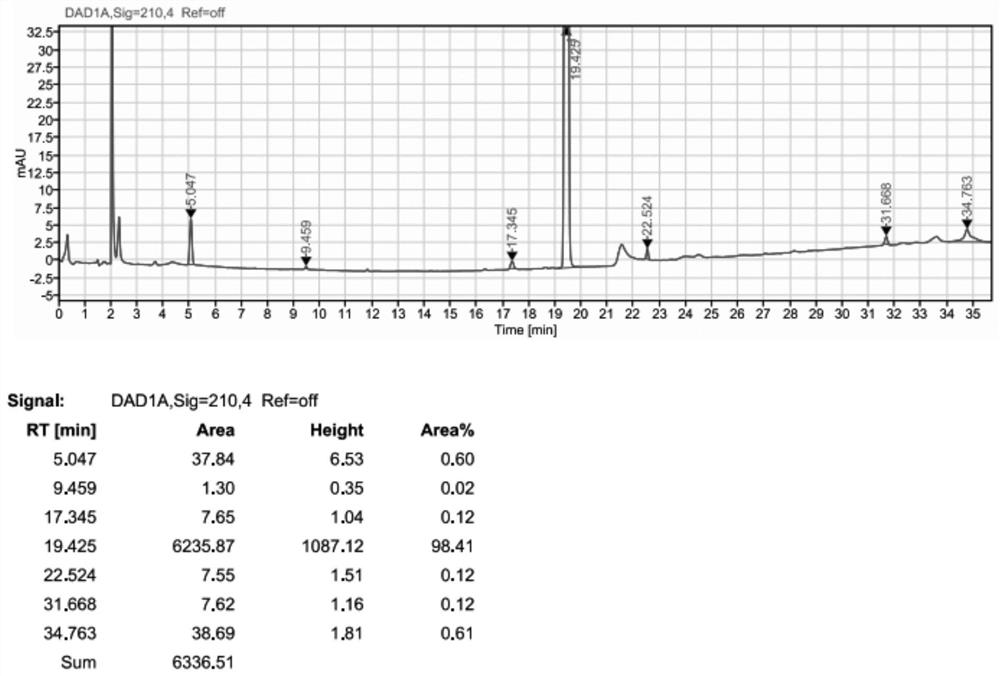
[0099] Example 3 BOC- (R) -3-amino-4- (2,4,5-trifluorophenyl) butyric acid condensation impurities
[0100]500 ml of four-mouth flask was input to 10 g (0.03 mol), (0.03 mol), (R) -3-amino-4- (2) (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) -3-amino-4- (2) 4,5-trifluorophenyl) butyric acid 8 g (0.0297 mol), HOBT 5g (0.036 mol), EDC-HCl 7g (0.036 mol), and 90 g of acetonitrile, the solid was dispersed uniform. Pyridine 3.16 g (0.04 mol) was added dropwise, the temperature was 20-30 ° C, and the insulation reaction was controlled until the end point. The acetonitrile was recovered under reduced pressure to give a solid crude material. The concentrate was purified by silica gel column (dichloromethane: ethyl acetate = 8: 1) Collect eluent containing impurities, concentrated to dryness to give a white solid. The solid was 9.86 g, the purity was about 99%, and the yield was about 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com