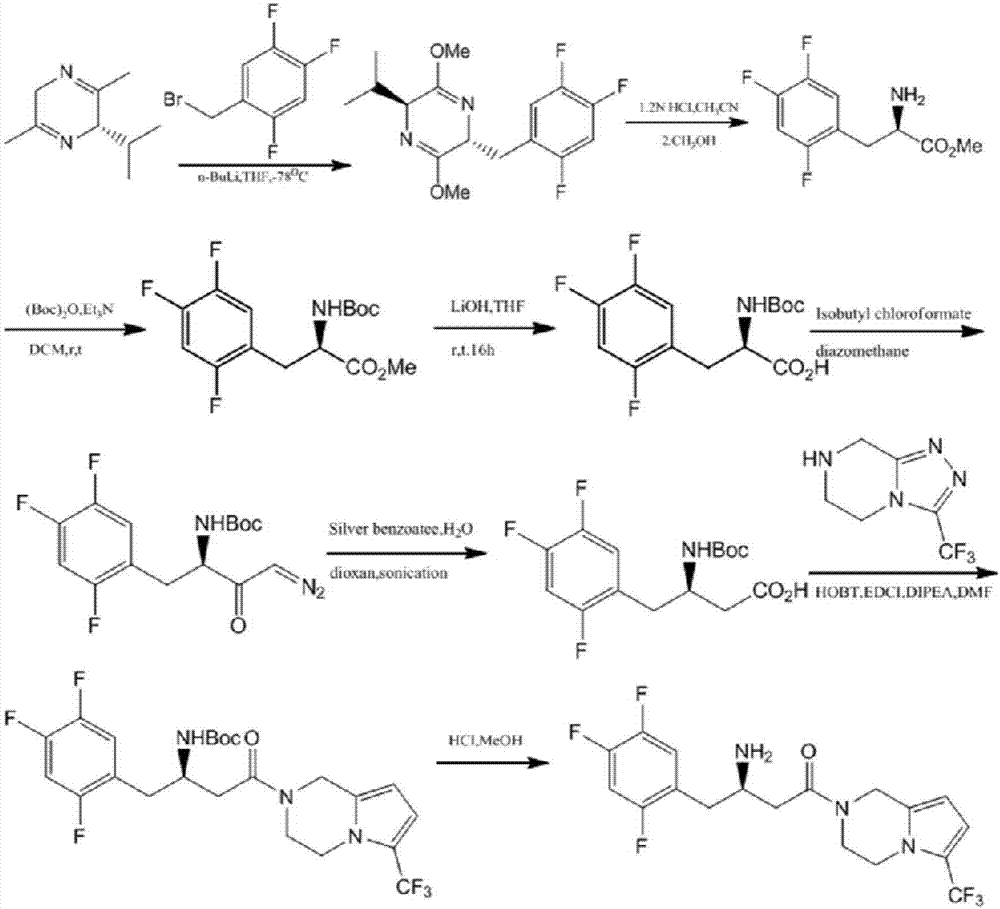
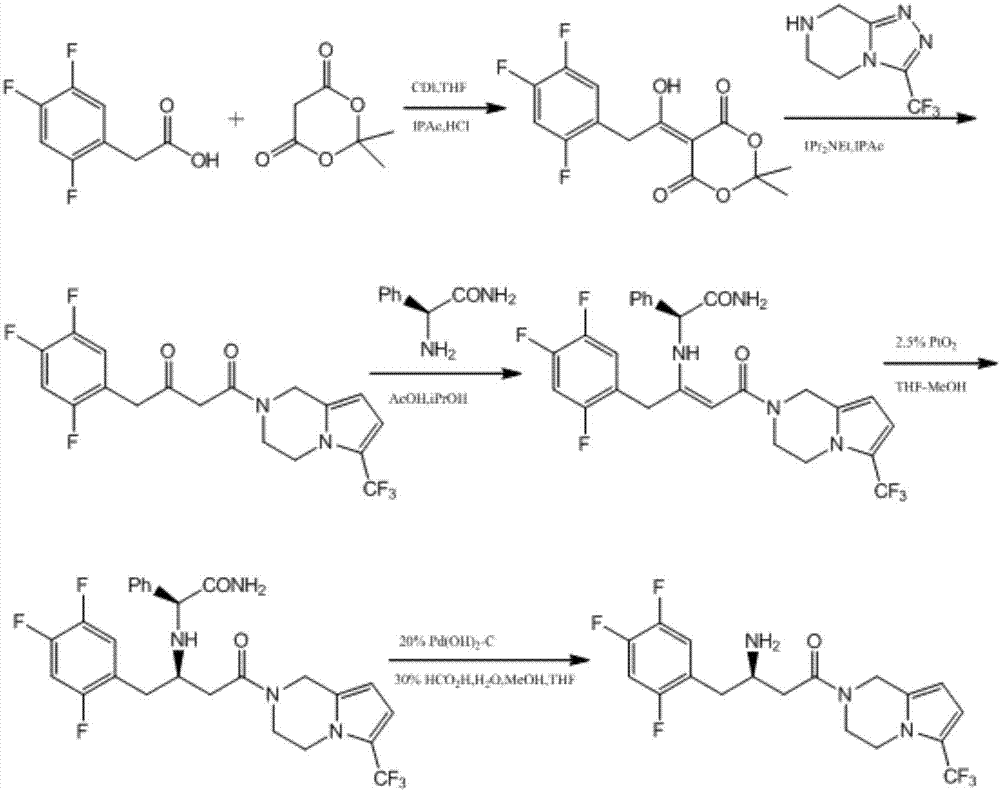
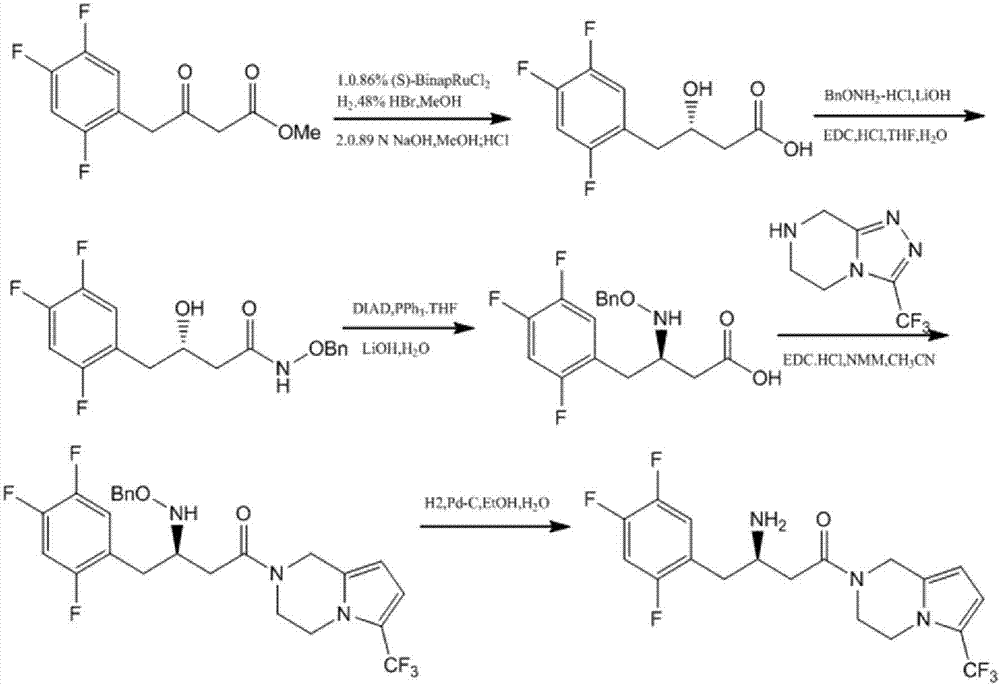
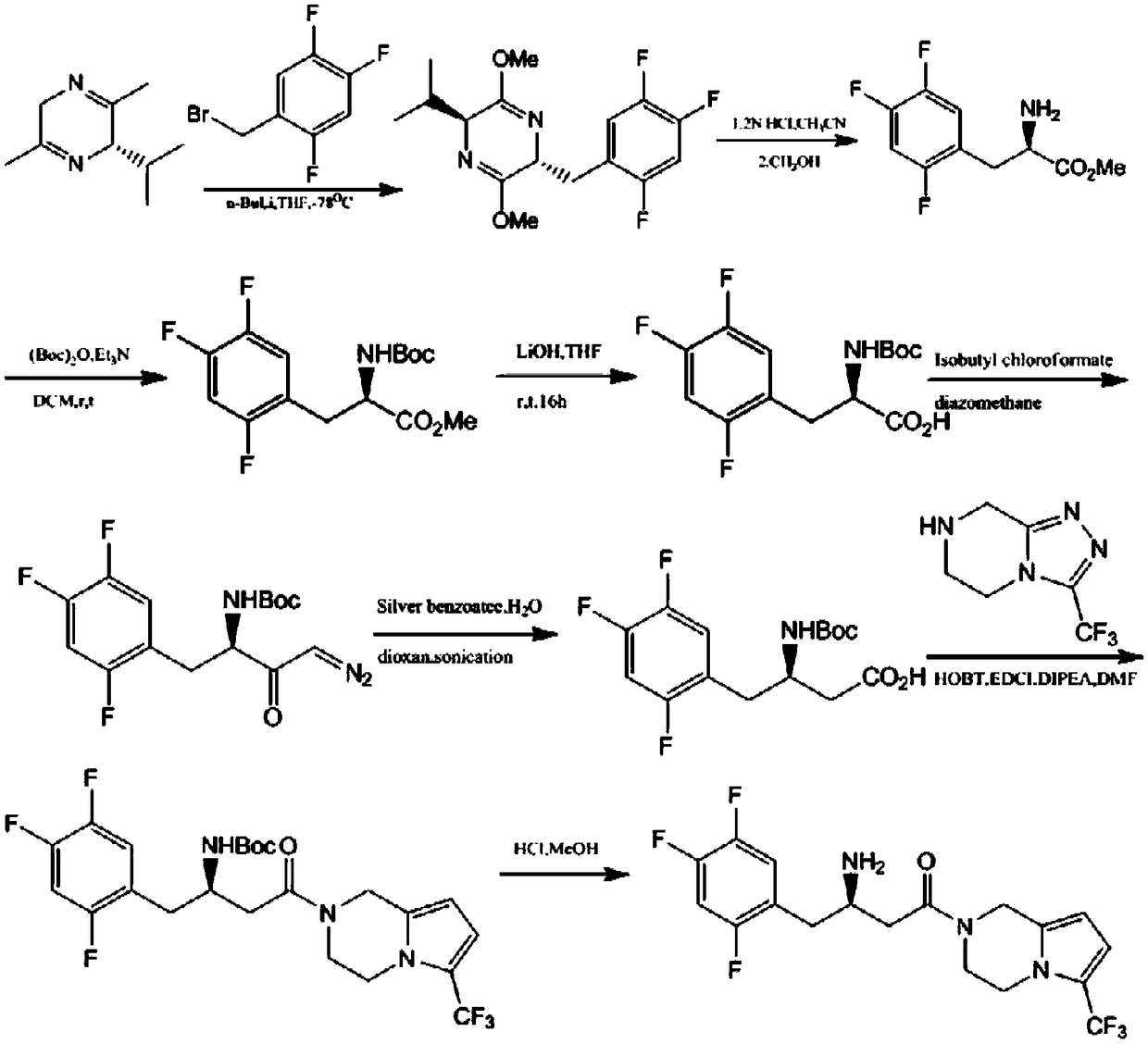
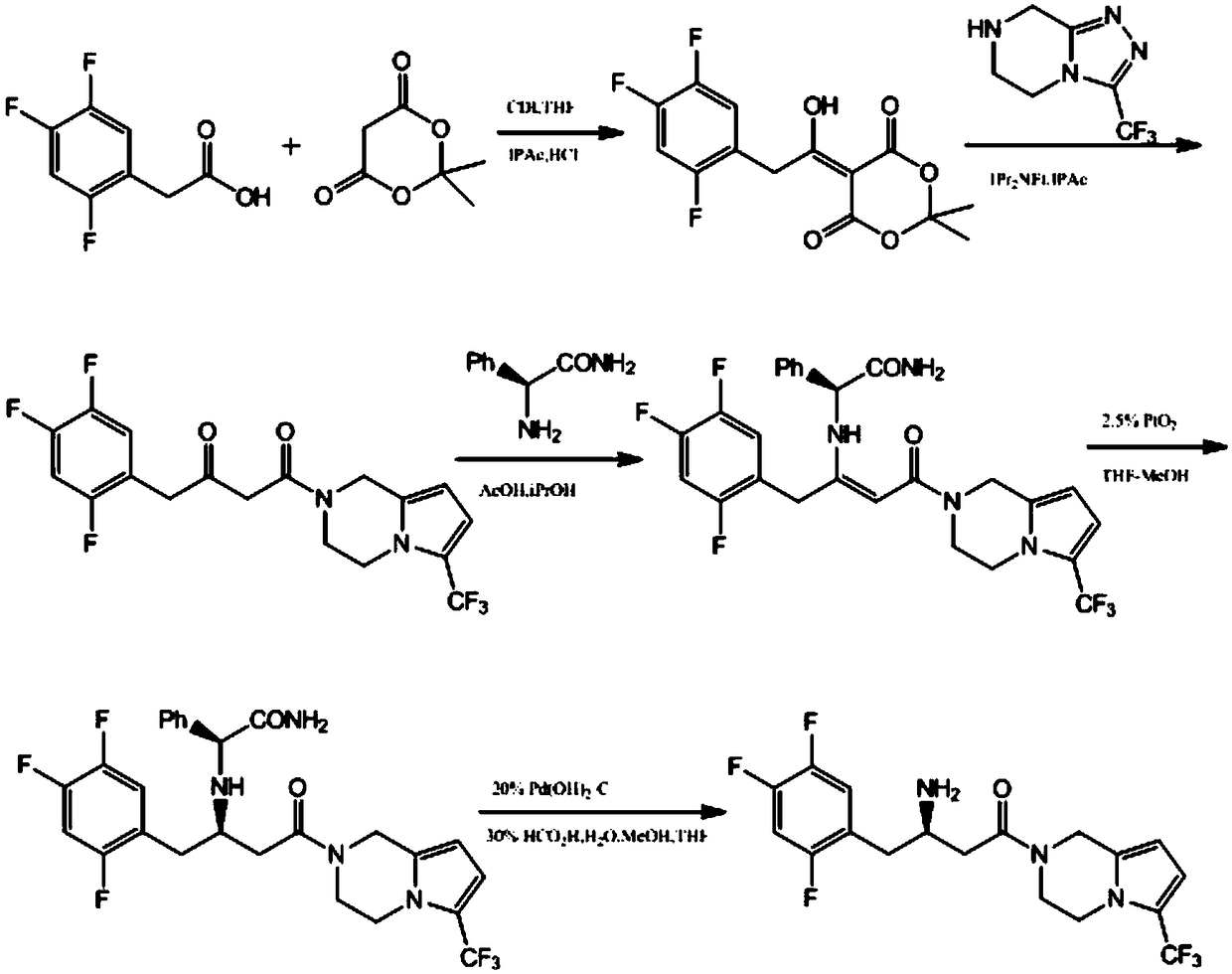
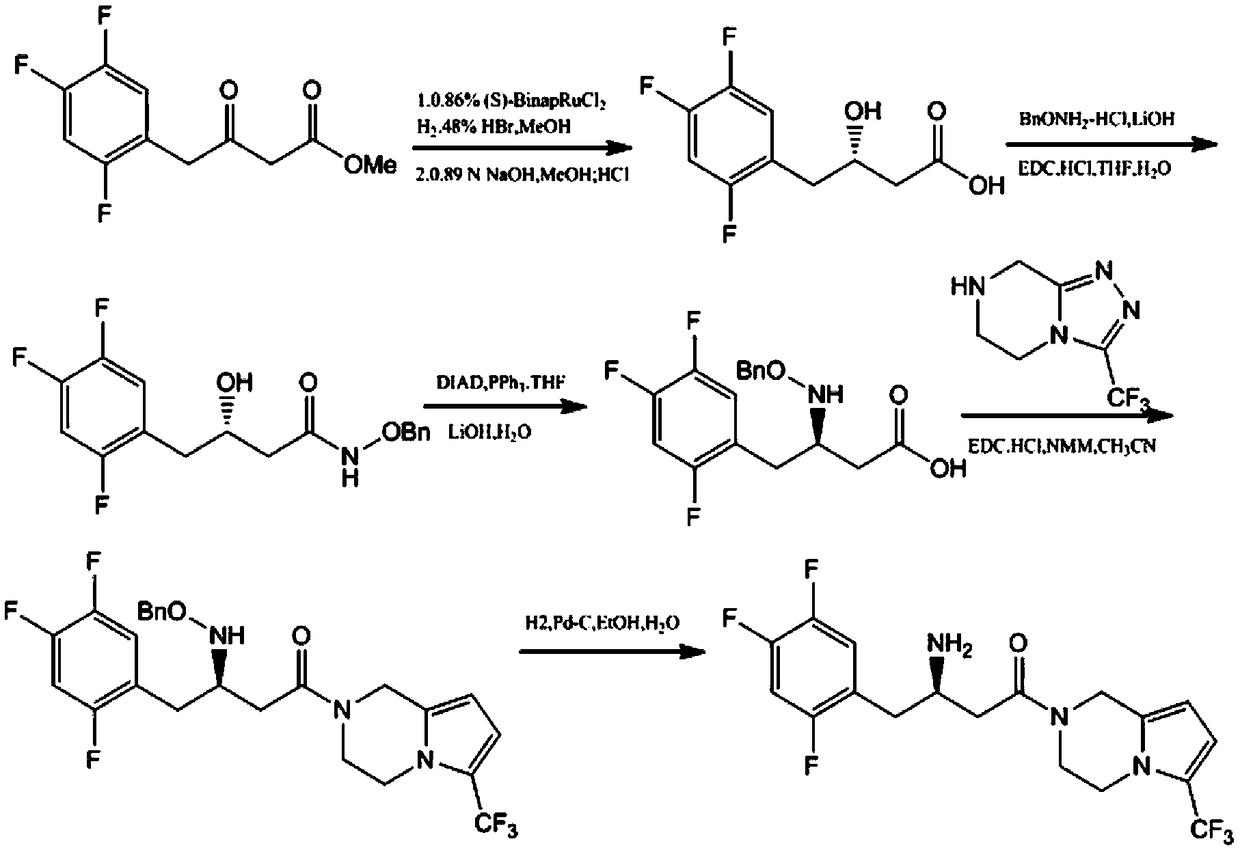
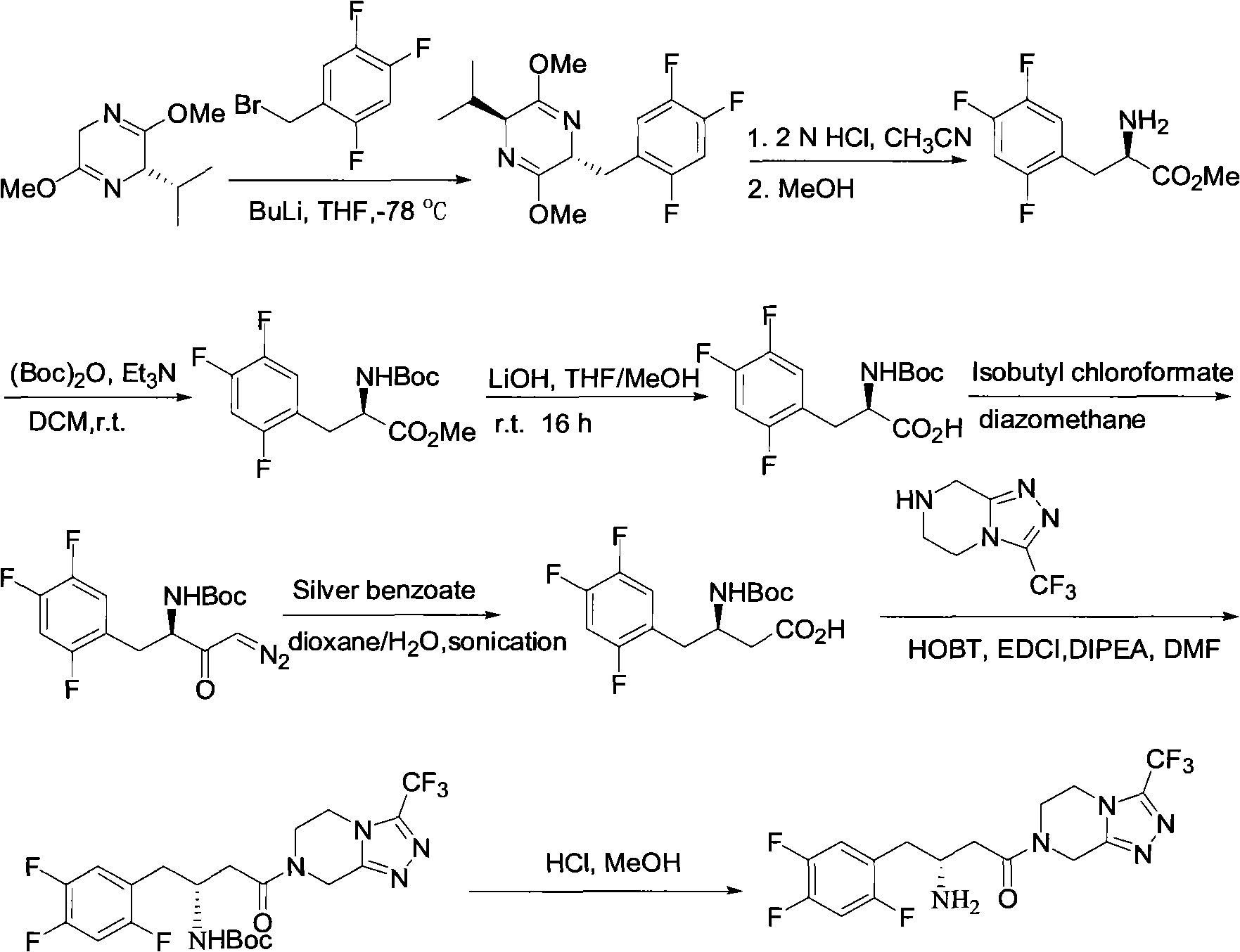
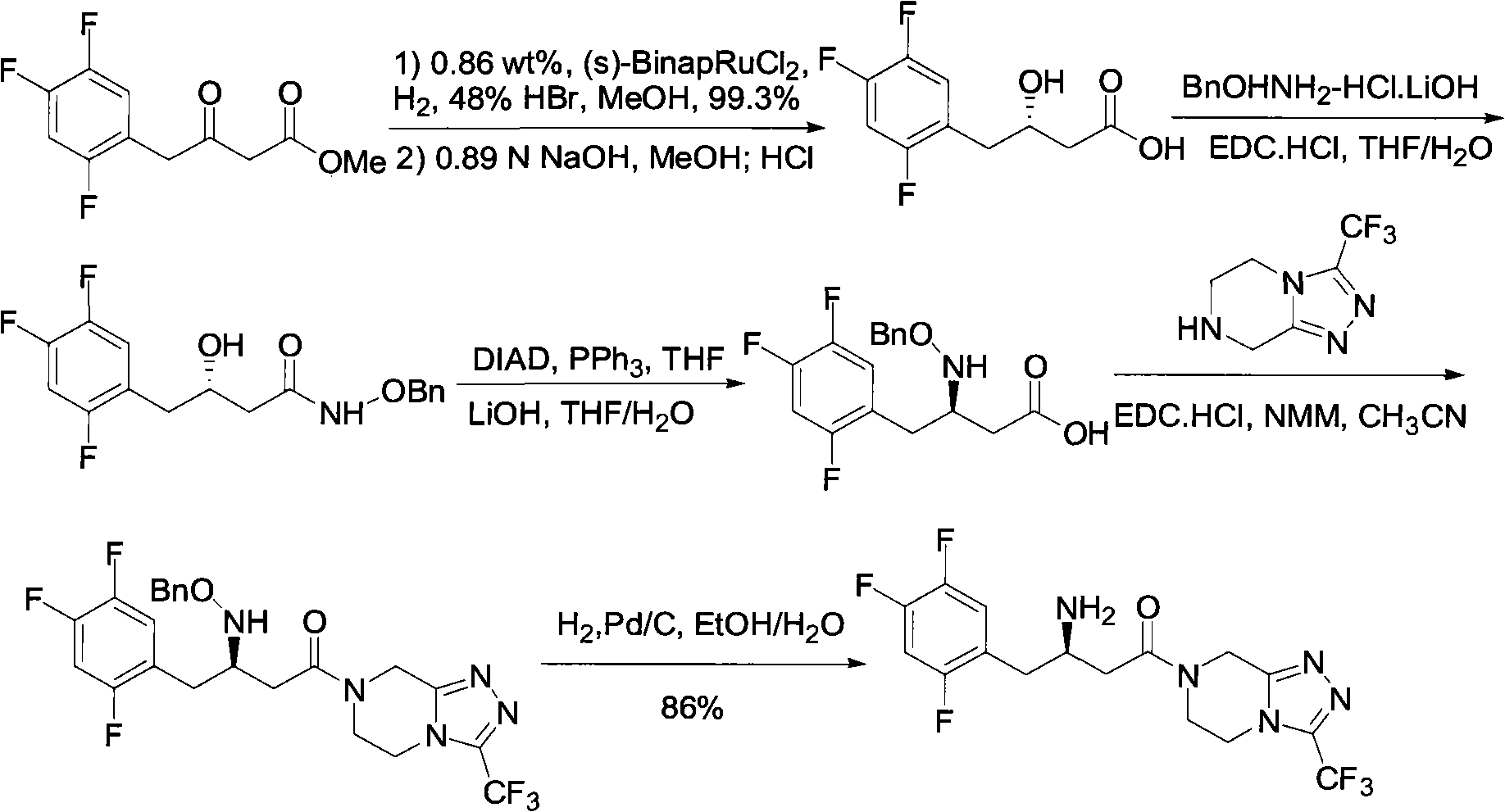
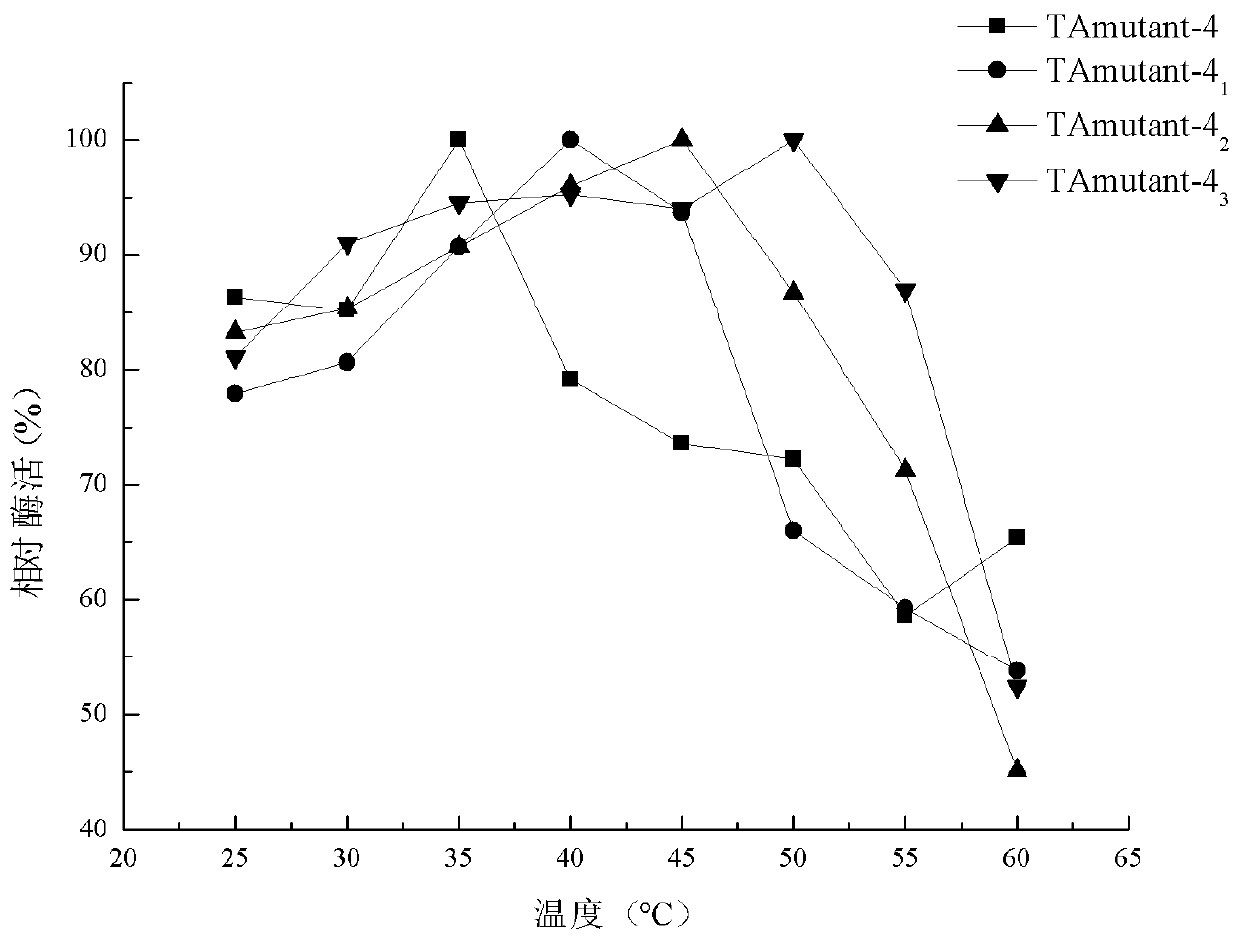
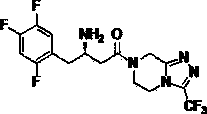
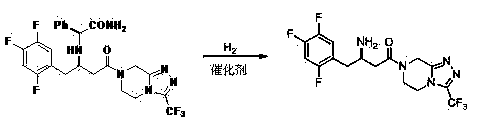
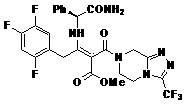
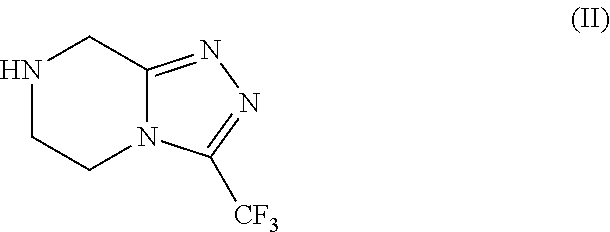
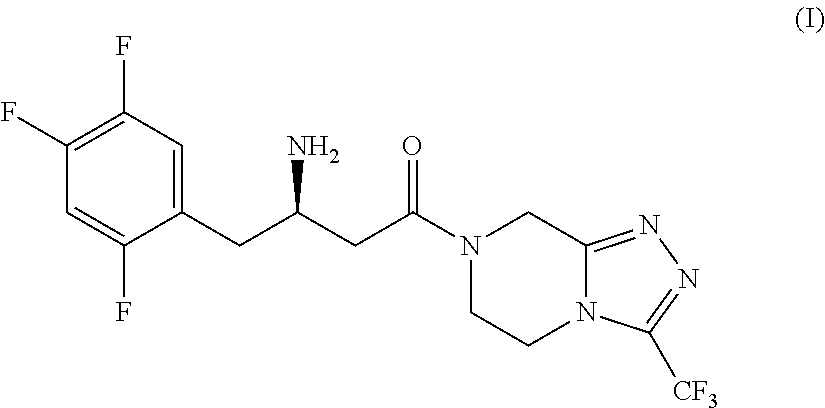
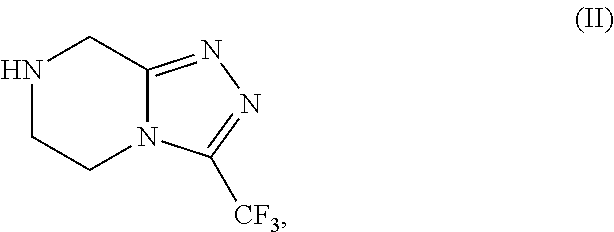
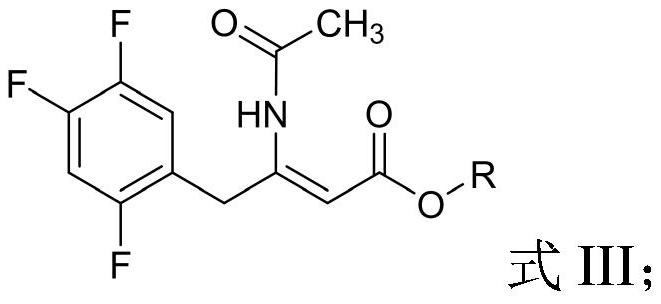
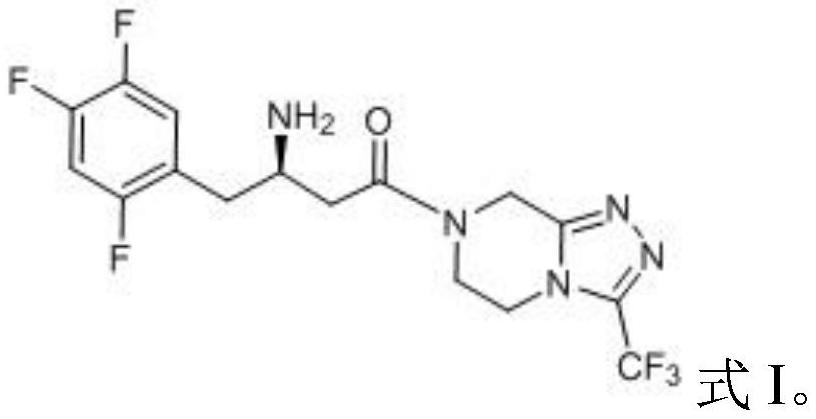
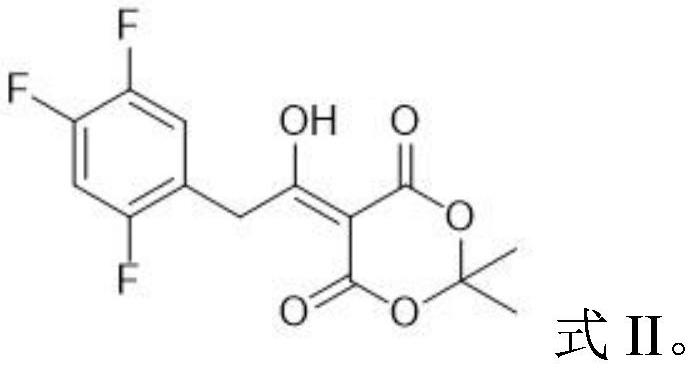
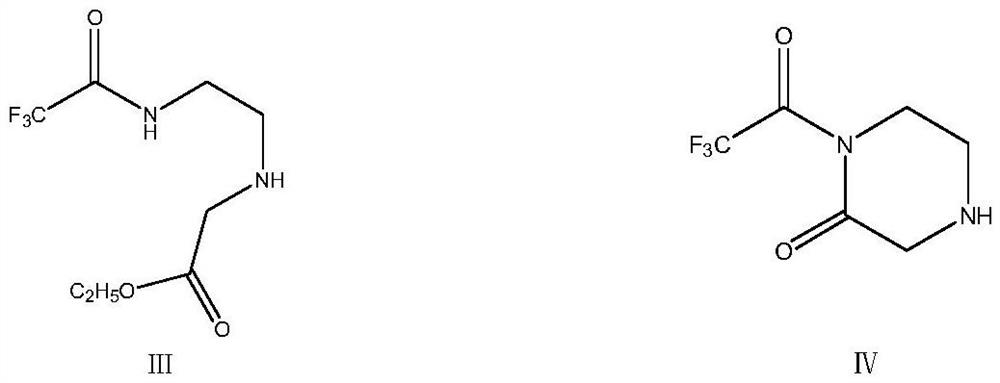Patents
Literature
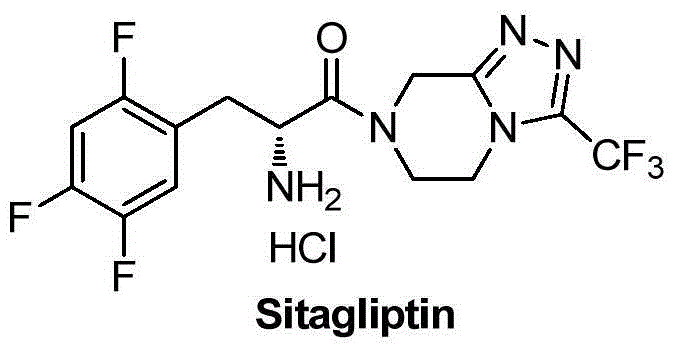
85 results about "Sitagliptina" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
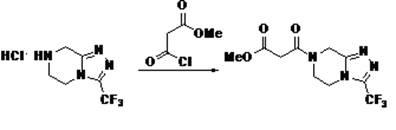
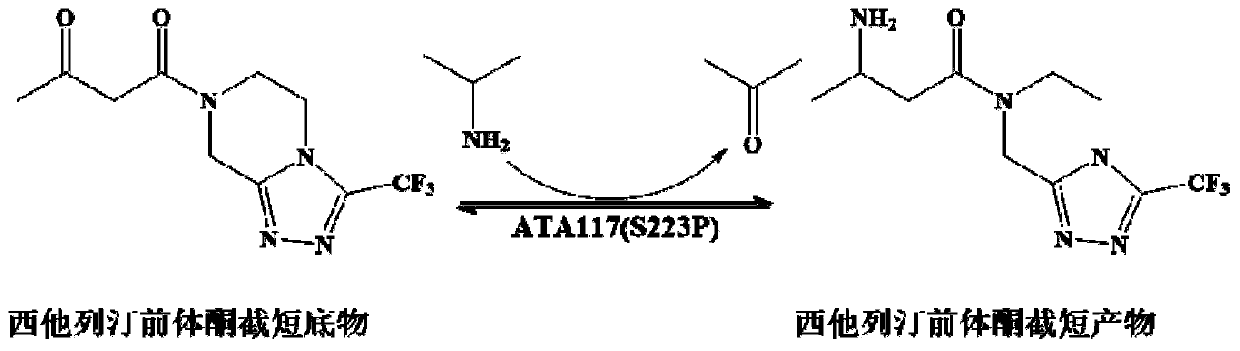
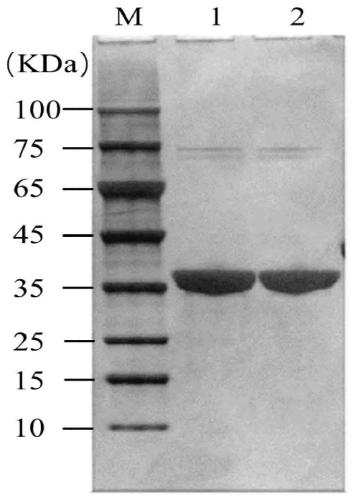
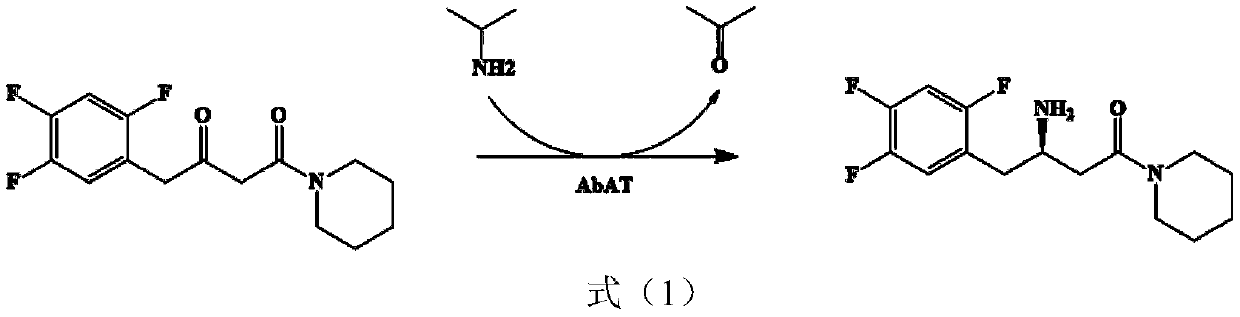
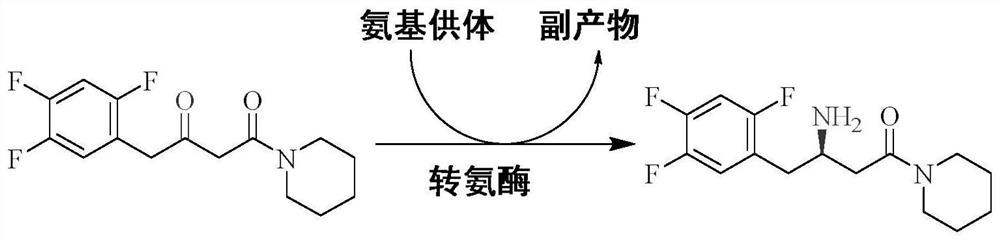
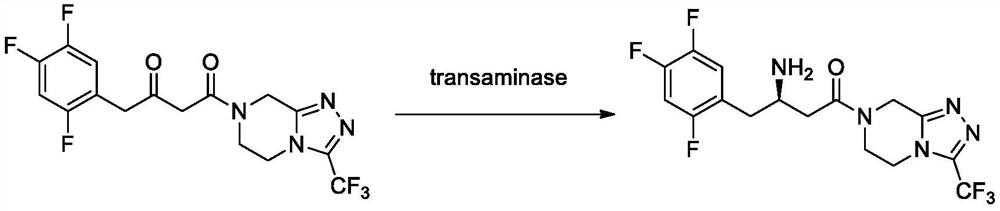
Aminotransferase, mutant and application to Sitagliptin preparation
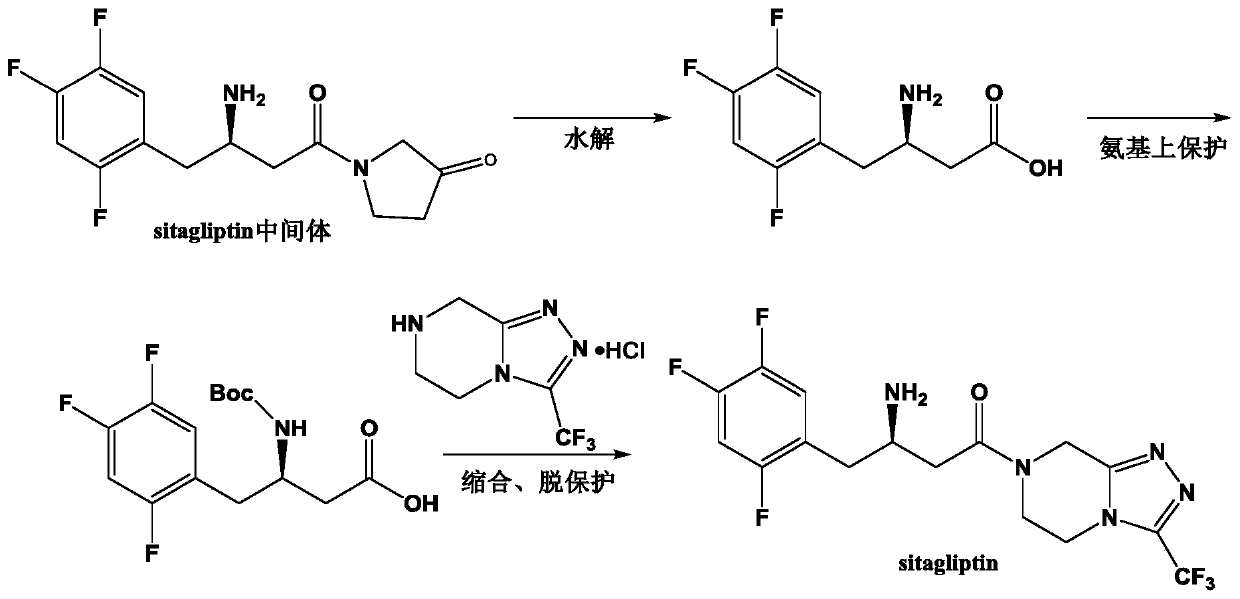
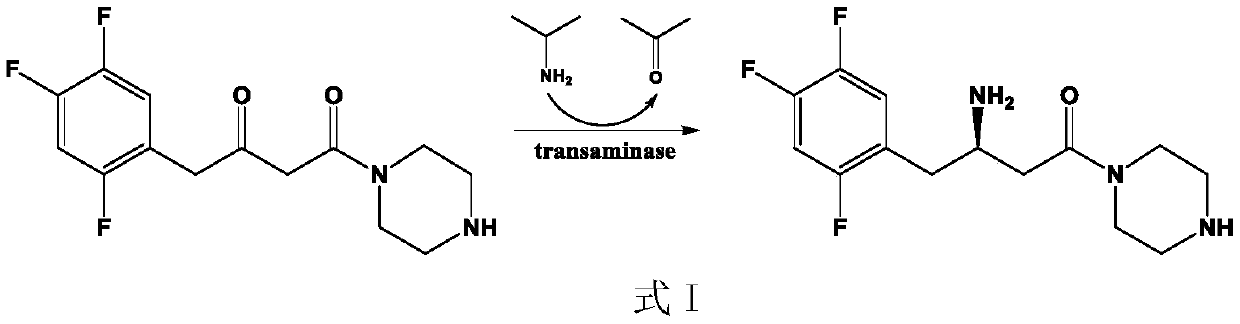
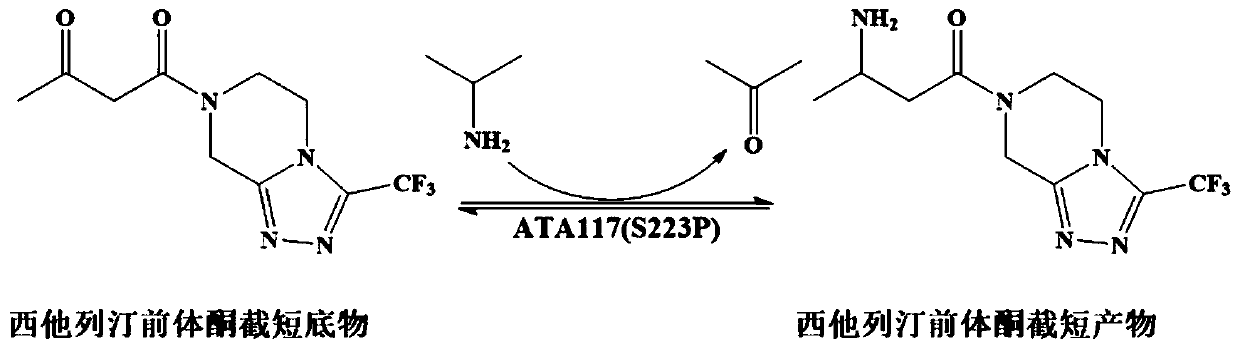
The invention discloses aminotransferase, a mutant and application to Sitagliptin preparation. According to the application, wet thalli obtained by performing fermentation culture on recombinant escherichia coli containing aminotransferase encoding genes are used as biocatalysts; Sitagliptin precursor ketone is used as a substrate; dimethyl sulfoxide is used as a latent solvent; phosphopyridoxal is used as a coenzyme; isopropylamine is used as an auxiliary substrate; a trolamine buffer solution with the pH being 8 to 9 is used as a reaction medium; a reaction system is formed; the biocatalytic reaction is performed under the conditions of the temperature being 30 to 45 DEG C and the stirring speed being 100 to 250 r / min; after the reaction is completed, the reaction liquid is separated and purified; the Sitagliptin is obtained. The aminotransferase and the mutant are used as biocatalysts; the latent carbonyl compound of Sitagliptin precursor ketone is directly used as the substrate; meanwhile, biocatalytic reaction is performed by using isopropylamine as the auxiliary substrate and using the pyridoxal phosphate as the coenzyme; the separation and purification is performed; Sitagliptin with high optical purity is prepared. The method has the advantages that the total yield is 76 percent; the product e.e. value reaches 99 percent.
Owner:ZHEJIANG UNIV OF TECH +2
Transaminase mutant as well as application thereof to preparation of sitagliptin midbody
The invention discloses a transaminase mutant as well as application thereof to preparation of sitagliptin midbody. The application adopts a wet thallus obtained by fermenting and culturing recombinant escherichia coli comprising aminotransferase coded gene as a biological catalyst, adopts sitagliptin midbody precursor ketone as a substrate, adopts dimethyl sulfoxide as a co-solvent and , adopts phosphopyridoxal as co-enzyme, adopts isopropylamine as an auxiliary substrate, adopts a pH 8-9 triethanolamine buffering solution as a reaction medium to form a reaction system to perform the biological catalytic reaction under the conditions that the temperature is 30 to 45 DEG C, and the stirring rate is 100 to 250 r / min, after the reaction is ended, the reaction solution is separated and purified to obtain sitagliptin; and the total yield of the method is about 81 percent, and a e.e. value of the product reaches 99 percent.
Owner:ZHEJIANG UNIV OF TECH +2
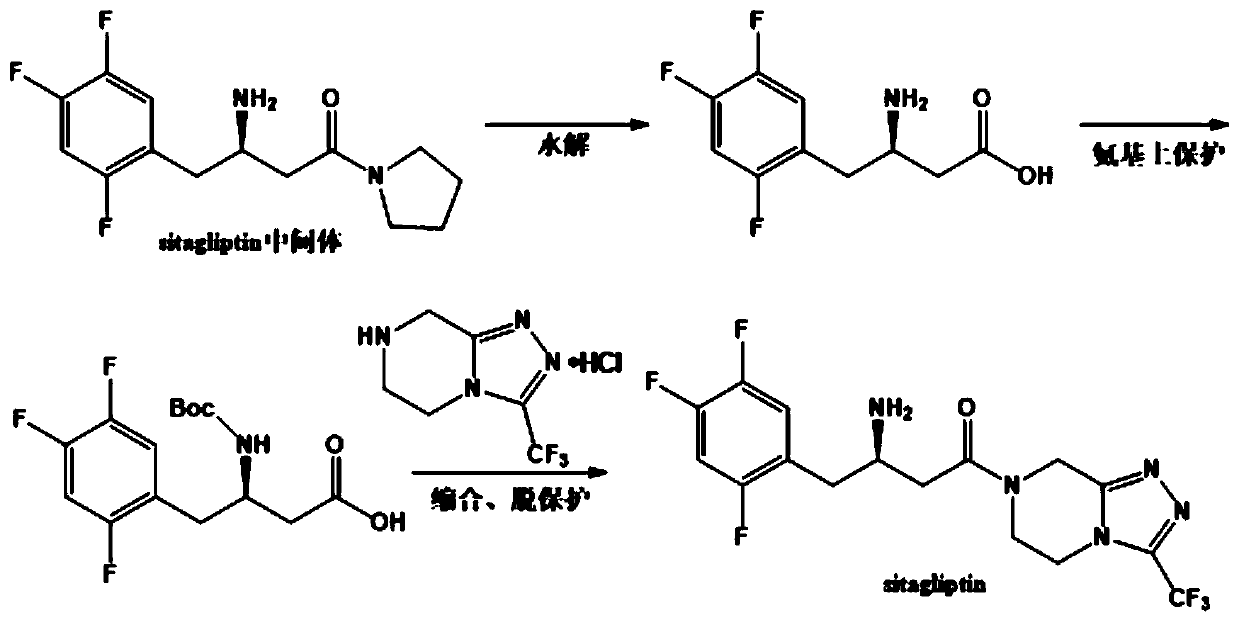
Sitagliptin intermediates as well as preparation method and application of intermediate
InactiveCN102838511AAvoiding asymmetric catalytic hydrogenation reactionsImprove economyPreparation by cyanide reactionPreparation from nitrilesSitagliptinEpoxy
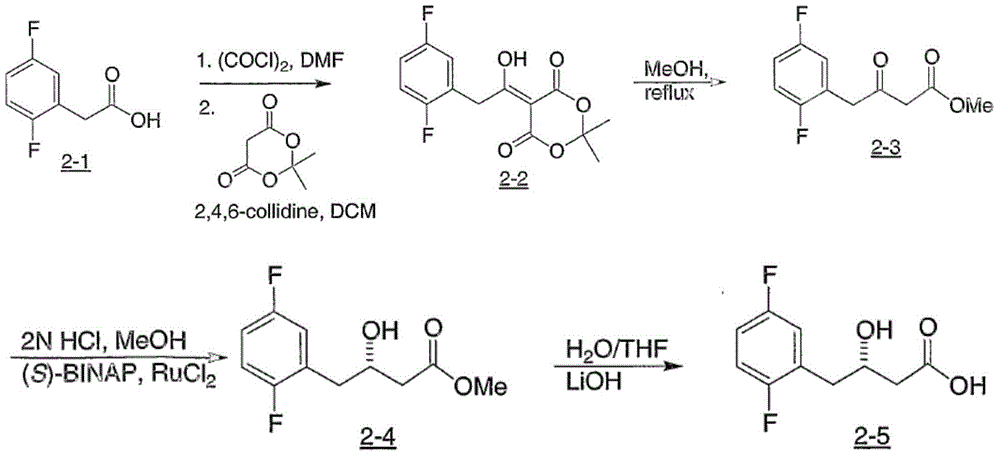
The invention relates to a Sitagliptin intermediates and a preparation method thereof as well as application of the intermediates in a method for preparing the Sitagliptin. According to the invention, epoxy chloropropane which is low in price and easy to obtain is used as a raw material to synthesize intermediates (S)-(2, 4, 5-trifluorophenyl) epoxy propane and (S)-3-hydroxy-4-(2, 4, 5-trifluorophenyl) butyronitrile, thus a chiral center is introduced, the use of various complex chiral reagents are avoided, and the chiral asymmetric catalytic hydrogenation reaction can be avoided; and the preparation method has the advantages of simple synthetic route, environment conservation, low raw material cost, etc.
Owner:ZHEJIANG HISOAR PHARMA
Process for preparing an intermediate of sitagliptin via enzymatic conversion
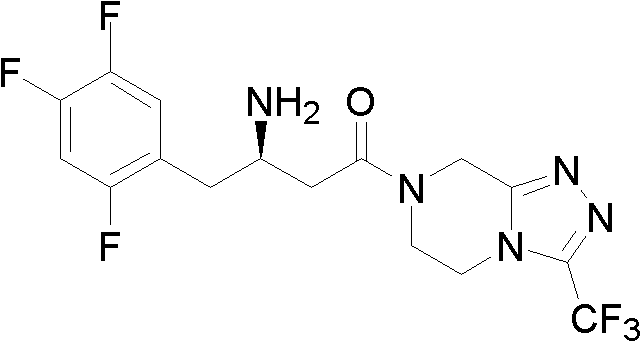
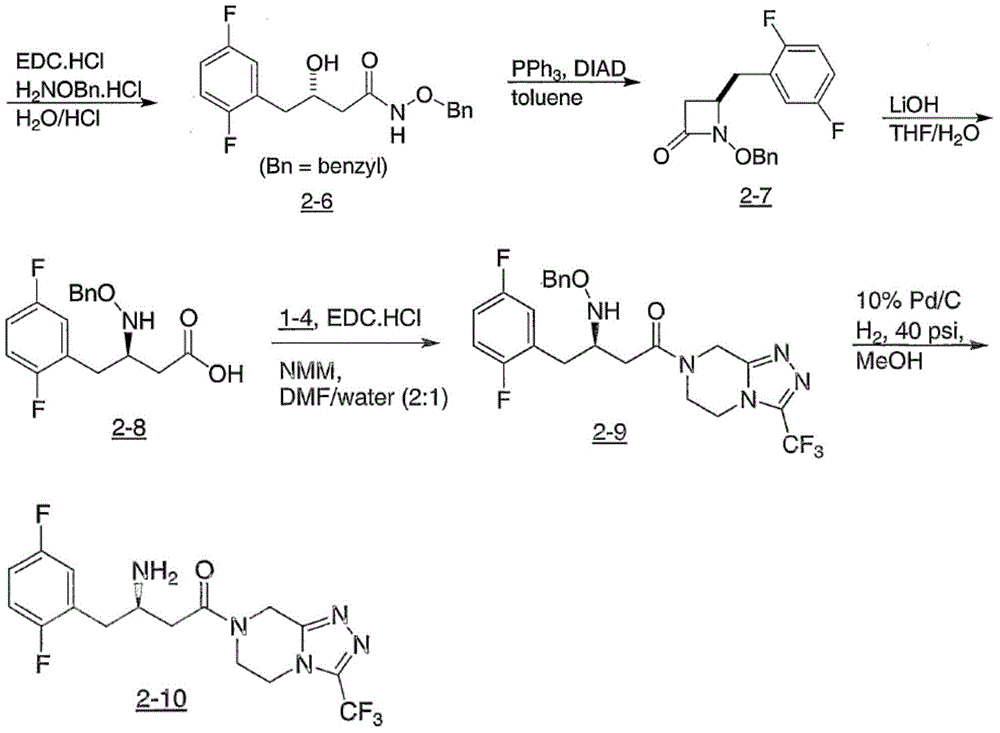
The invention provides a process for preparing 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one (Formula I), into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms comprising: a) reacting 4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-one of formula (III) with a suitable oxidoreductase enzymes or its suitable variants in the presence of suitable conditions and co-factor; and b) isolating 3-hydroxy-1-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-4-(2,4,5-trifluorophenyl)butan-1-one, into its racemic (R / S) form or any of its optically active (S) or (R) forms or enantiomeric excess mixture of any of the forms.
Owner:CADILA HEALTHCARE LTD
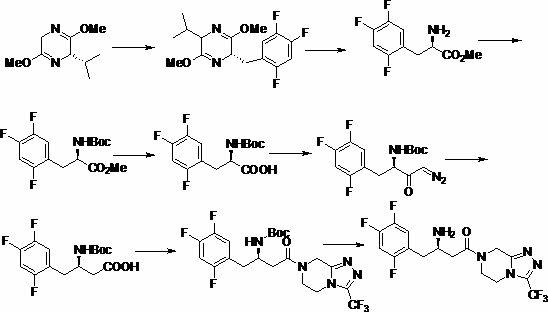
Intermediates of Sitagliptin and preparation method thereof
ActiveCN102126976ASimple processReduce dosageOrganic compound preparationAmino-carboxyl compound preparationSitagliptinChemical structure
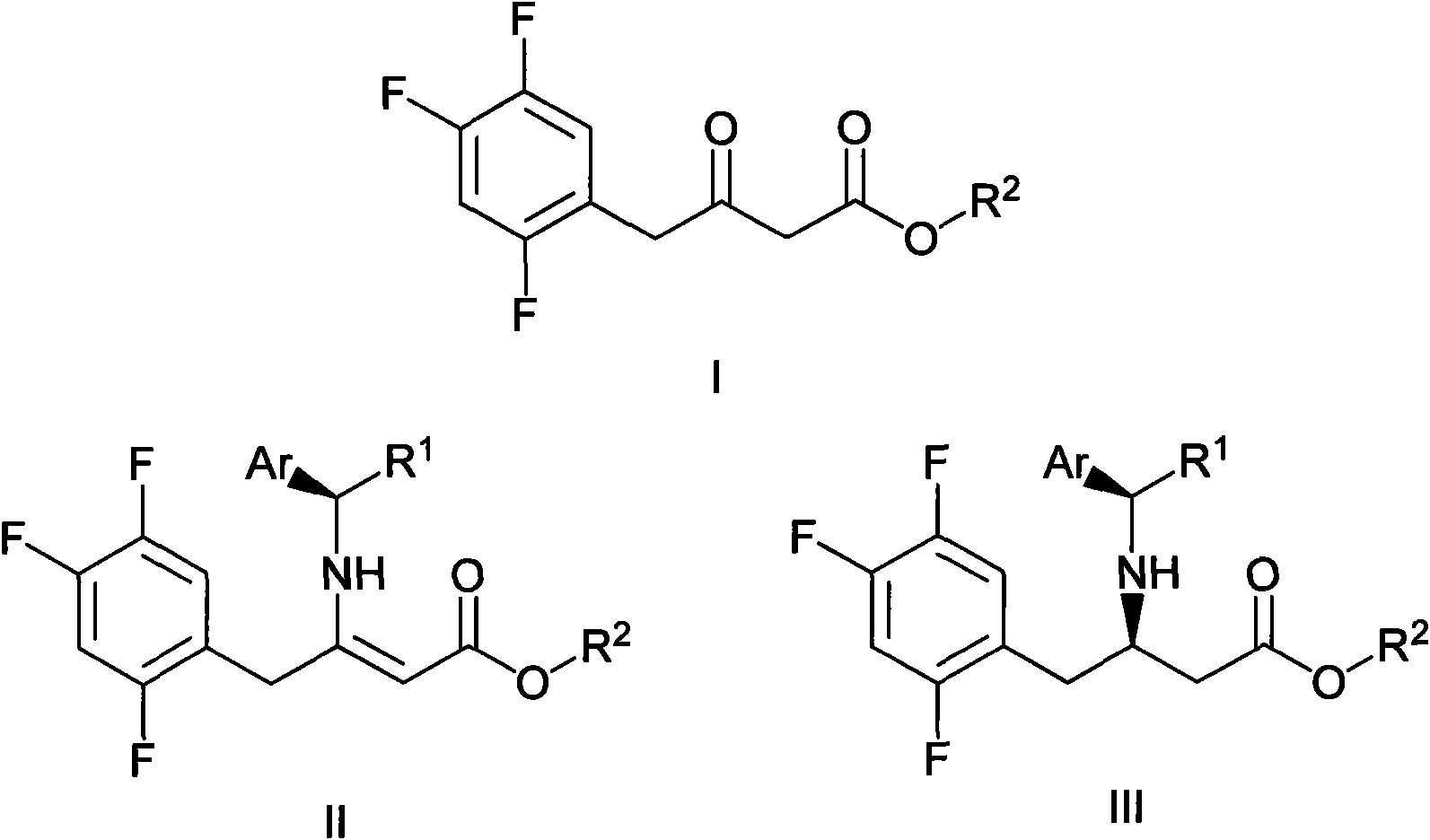
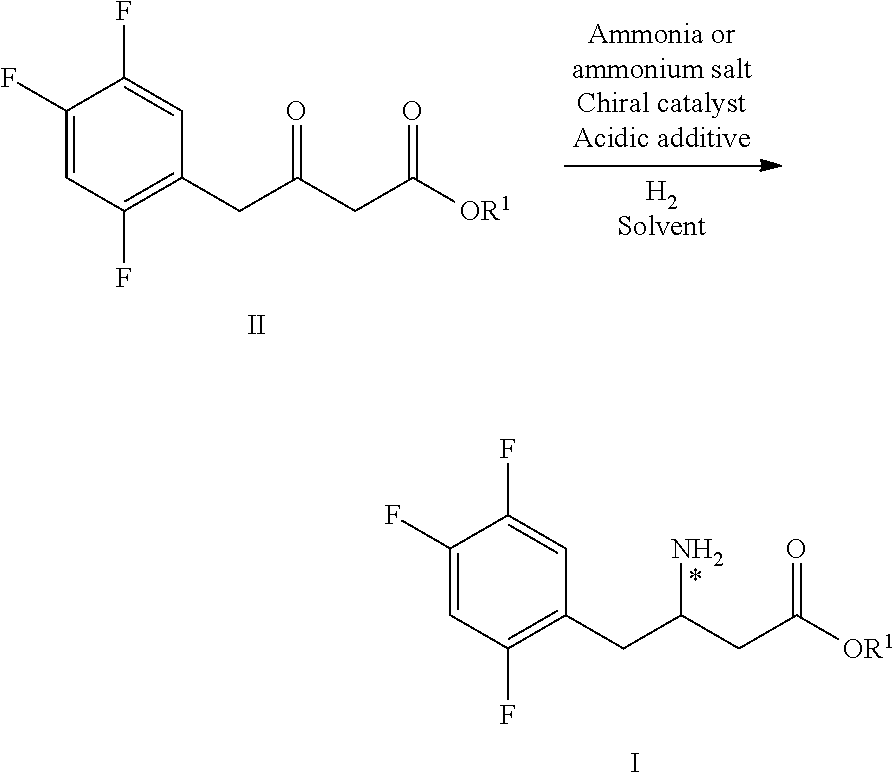
The invention mainly relates to intermediates of Sitagliptin and a preparation method thereof. The intermediates of the Sitagliptin have chemical structures shown as II and III, wherein definitions of Ar, R1 and R2 are shown in the specifications. The compounds shown as the II and the III are prepared by reacting a compound shown as a formula I with a substituent of phenylethylamine, which serve as raw materials, under hydrogen pressure. The intermediates are used for synthetizing the Sitagliptin, so synthetic difficulty is reduced, purity of a product is improved, the using amount of a high-price catalyst is reduced, production cost is effectively reduced, and the intermediates and the method are suitable for industrialized production.
Owner:JIANGSU SENRAN CHEM +1
Aspartase mutant and applications thereof
ActiveCN111041019AImprove conversion rateHigh stereoselectivityCarbon-nitrogen lyasesBacteriaButyrateMutant
The invention discloses an aspartase mutant, which comprises the following mutations M321V, K324T and N326S on an amino acid sequence represented by SEQ ID NO.2, and has high enzymatic activity compared with wild-type aspartase in catalysis of (E)-4-(2,4,5-trifluorophenyl)2-butenoic acid. The invention also discloses applications of the aspartase mutant in preparation of (R)-3-amino-4-(2,4,5-trifluorophenyl)-butyric acid. According to the invention, with the application of the aspartase mutant in an enzyme catalytic reaction to prepare a sitagliptin chiral intermediate (R)-3-amino-4-(2,4,5-trifluorophenyl)-butyric acid, the conversion rate is high, the stereoselectivity is high, the yield is high, the production cost is low, the environment is friendly, and industrial large-scale production is facilitated.
Owner:ABIOCHEM BIOTECH CO LTD
Preparation method of sitagliptin
ActiveCN102627648ALow costHigh yieldOrganic chemistryBulk chemical productionPhenylacetic acidMethyl palmoxirate
The invention provides a preparation method of sitagliptin. The preparation method comprises the following steps of: performing condensation reaction on hydrochloride of 3-trifluoromethyl-[1,2,4] triazol [4,3-a] piperazine serving as a starting raw material and methyl malonyl chloride under a normal temperature condition; reacting an obtained product with 2,4,5-trifluorophenylacetic acid under an alkaline condition and then performing condensation reaction with (S)-phenylglycinamide under normal temperature condition to obtain a product; reducing the obtained product through a reducing agent; removing an ester group through heating reflux; and reacting with a hydrogenation reducing reagent to obtain the sitagliptin. The preparation method has the advantages of low cost, high yield, easiness in operation, all used reagents of conventional reagents, simple post-treatment and convenience for industrial production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
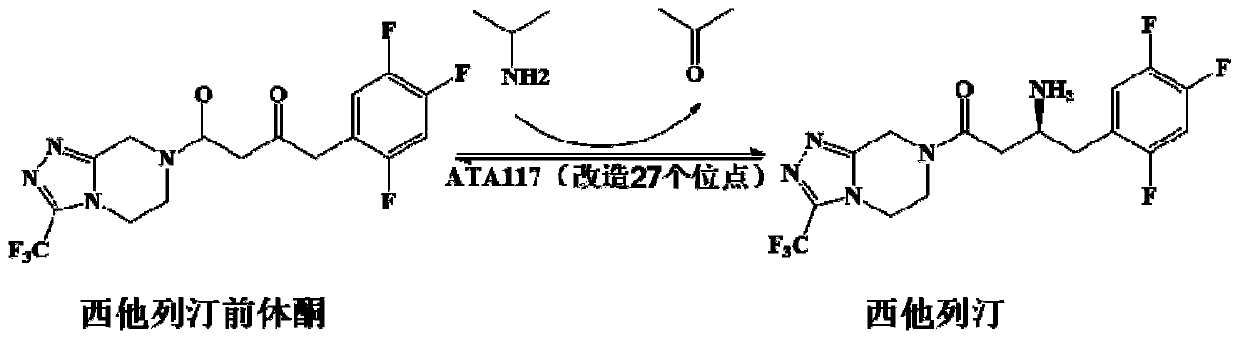
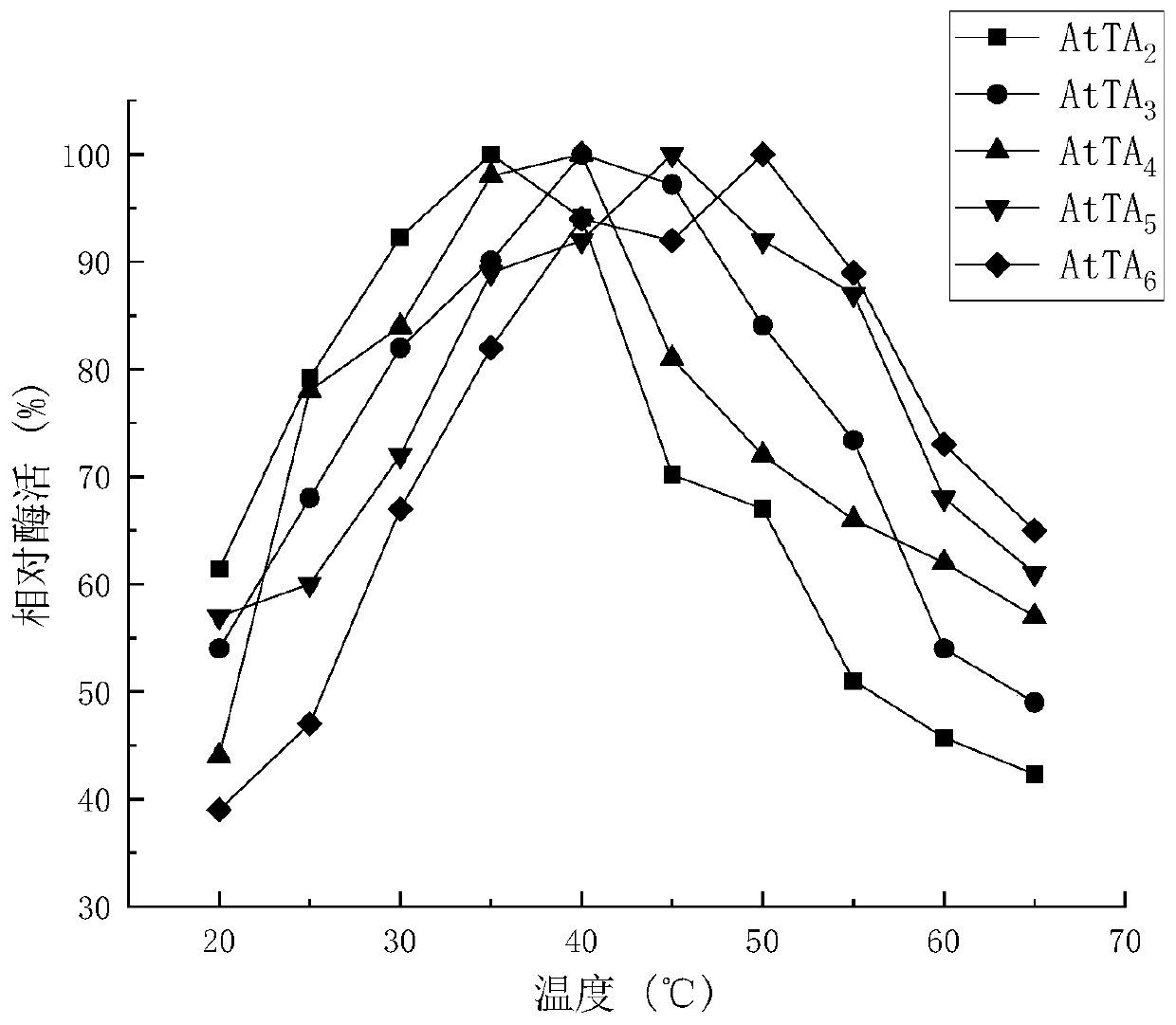
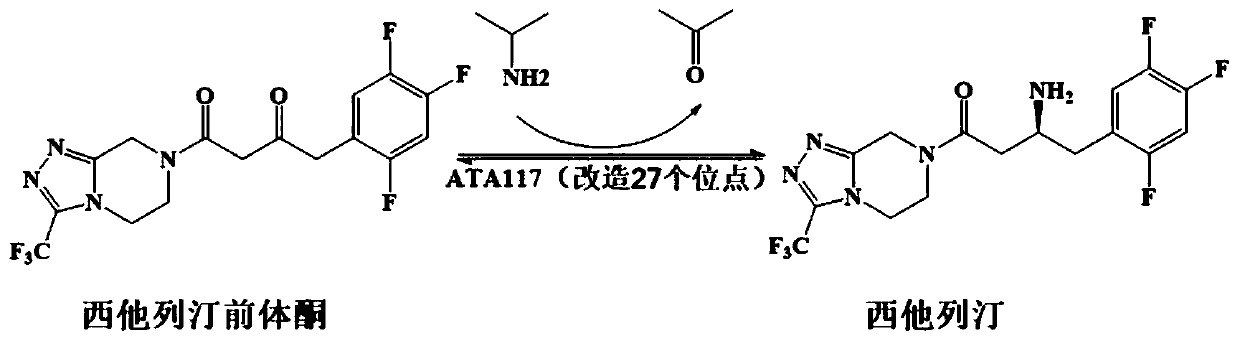
Recombinant R-Omega-transaminase, mutant and application of recombinant R-Omega-transaminase and mutant in asymmetrically synthesizing sitagliptin
ActiveCN110951706AHigh activityIncreased substrate toleranceTransferasesFermentationSitagliptinHigh activity
The invention relates to application of novel recombinant R-Omega-transaminase and a high-activity mutant of the recombinant R-Omega-transaminase in asymmetrically synthesizing sitagliptin by catalyzing sitagliptin precursor ketone. The amino acid sequence of the recombinant R-Omega-transaminase is shown by SEQ ID:1, and the mutant of the recombinant R-Omega-transaminase is obtained by performingsingle-site mutation or multisite mutation on one or more of a 60th site, 113th site, 178th site, 233th site, 146th site, 214th site and 186th site of the amino acid sequence shown by SEQ ID:1. The mutant of the novel R-Omega-transaminase with high activity and high stereoselectivity is provided; the mutant has milestone significance in realizing autonomization and localization of a biological catalysis preparation technology for the sitagliptin; and the technology monopoly situation that an enzyme source is single in the process of asymmetrically synthesizing the sitagliptin at present can bechanged.
Owner:ZHEJIANG UNIV OF TECH
Reagent, method and kit for detection of biological activity of glucagon-like peptide-1 (GLP-1)
The invention relates to a reagent for detection of biological activity of glucagon-like peptide-1 (GLP-1). The reagent added into each 15 microliters of a detection sample comprises: 1, 35-45 microliters of a membrane reaction solution, 2, 15-25 microliters of a GLP-1 acceptor, 3, 10-20 microliters of a GLP-1 standard substance, 4, 5-15 microliters of sitgliptin phosphate, 5, 1-10 microliters of triphosadenine, 6, 1-10 microliters of guanosine triphosphate, 7, 5-15 microliters of 3-isobutyl-1-methylxanthine, 8, 1-10 microliters of bovine serum albumin, 9, 50-150 microliters of a biotin-labeled mouse anti-human cAMP monoclonal antibody, 10, a microwell plate coated with a goat anti-human cAMP polyclonal antibody, 11, 50-150 microliters of an avidin-labeled horseradish peroxidase, 12, 300-400 microliters of a PBS washing liquor, 13, 50-150 microliters of a chromogenic substrate, and 14, 50-150 microliters of a stop buffer. The GLP-1 acceptor is produced by genetic recombination, is convenient for purification and can reduce the batch difference of the reagent. The GLP-1 acceptor is used as a probe for capturing GLP-1 so that detection sensitivity is improved, biological activity can be directly determined, sensitivity is high, and practicality is strong.
Owner:MEIDE TAIPINGYANG PRECISION INSTR MFG
Preparation methods for sitagliptin intermediates
ActiveCN102503829AMild reaction conditionsSimple and fast operationOrganic compound preparationCarboxylic acid esters preparationGrignard reagentPyrazine
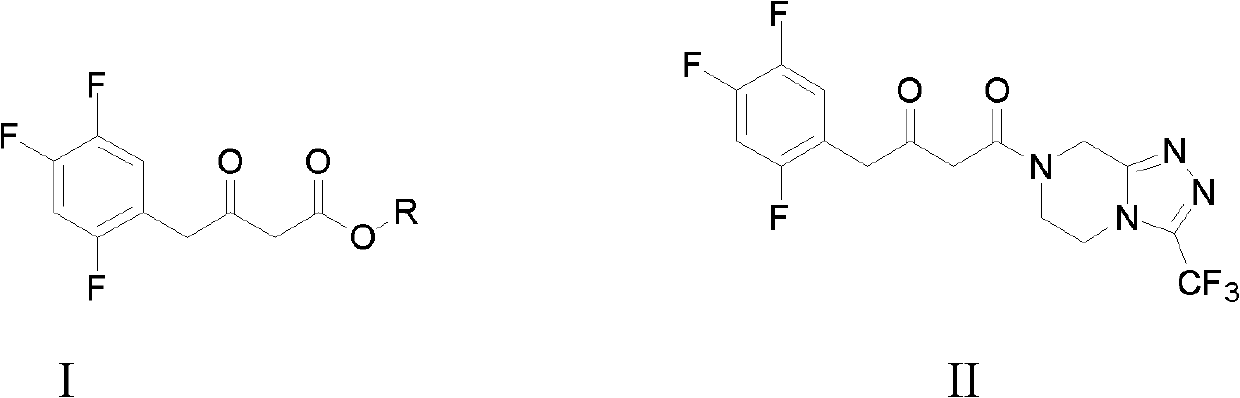
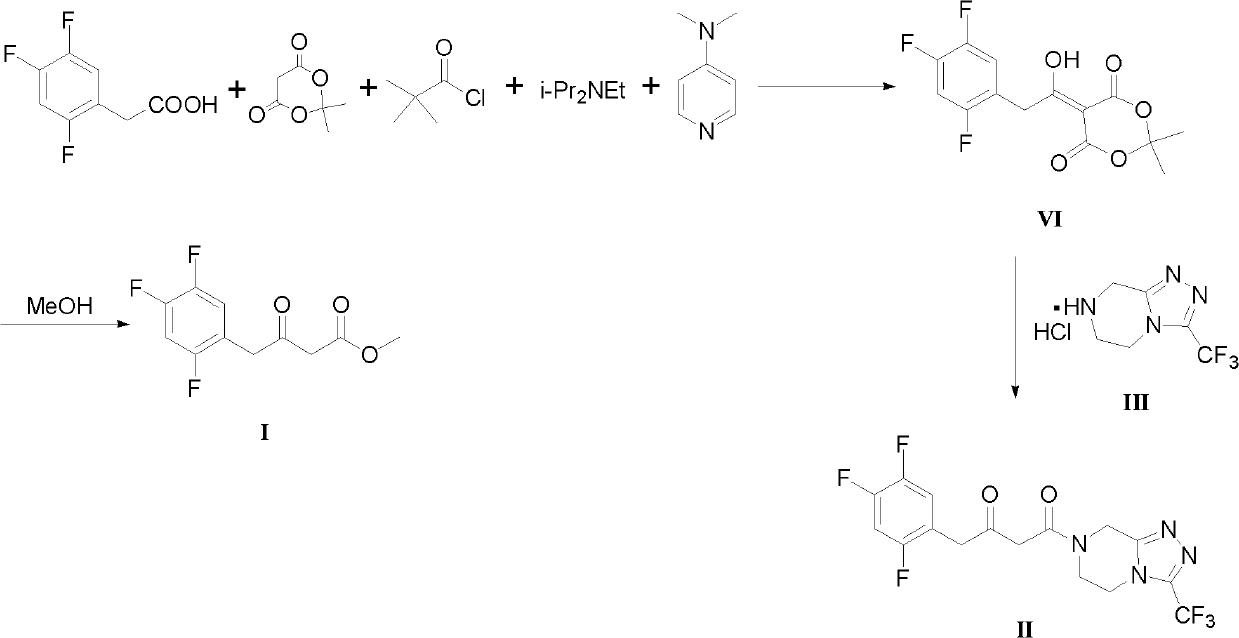
The invention discloses preparation methods for sitagliptin intermediates including 3-carbonyl-4-(2,4,5-trifluorophenyl)-butyrate (I) and 1-(3-trifluoromethyl-5,6-dihydro-8H-(1,2,4) triazole-(4,3-a) pyrazine-7-)-4-(2,4,5-trifluorophenyl)-1,3-butanedione (II). The preparation method for the intermediate (I) includes the steps of preparing corresponding Grignard reagent by bromoacetate and magnesium powder by means of Grignard reaction, and preparing the intermediate I by means of addition reaction between the Grignard reagent and 2,4,5-trifluoro-benzeneacetonitrile. The preparation method for the intermediate (II) includes the steps of carrying out acylation reaction between bromoacetic acid and III under the action of condensing agent so that IV is prepared, carrying out Grignard reaction between the IV and magnesium powder so that corresponding Grignard reagent V is prepared, and finally preparing the intermediate (II) via addition reaction between the Grignard reagent V and 2,4,5-trifluoro-benzeneacetonitrile in organic solvent. The preparation methods for the sitagliptin intermediates are mild in reaction conditions, simple and convenient in operation and available in raw materials and have excellent industrialized prospect.
Owner:SHANDONG BOYUAN PHARM CO LTD
Application of sitagliptin phosphate in preparation of medicament for preventing and treating febrile convulsion
InactiveCN102600161AActive ingredients are clearLow costOrganic active ingredientsNervous disorderFebrile convulsionsSide effect
The invention belongs to technical field of medicines and relates to application of sitagliptin phosphate in preparation of a medicament for preventing and treating febrile convulsion. Currently, the sitagliptin phosphate has obvious medicament effect of clinically treating the febrile convulsion, but various serious side effects can be produced to damage children, and the tolerance and the addiction can be produced after long-time use. The product disclosed by the invention is definite in active ingredients, lower in cost and easy to implement.
Owner:WUHAN UNIV
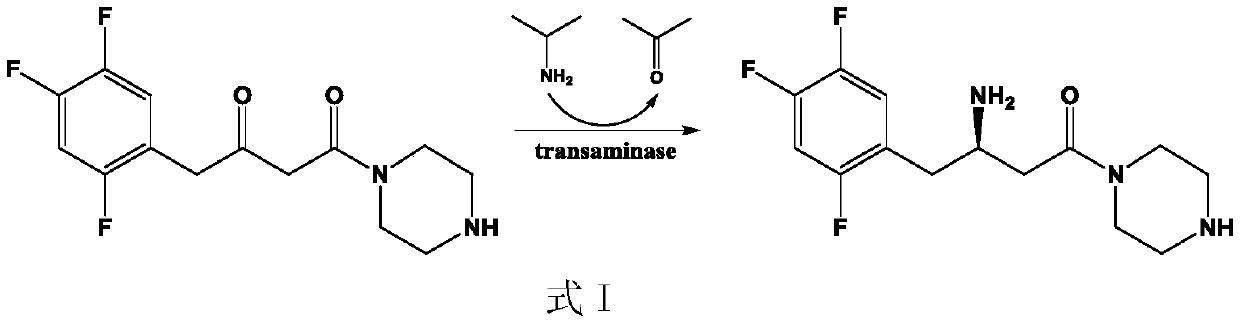
Amine transaminase AcATA mutant and application thereof in preparation of sitagliptin intermediate
ActiveCN111549008AIncrease enzyme activityImprove conversion rateBacteriaTransferasesKetonePhenylalanine
The invention discloses an amine transaminase AcATA mutant and application of the amine transaminase AcATA mutant in preparation of a sitagliptin intermediate. The amine transaminase AcATA mutant is obtained by single mutation at the 122th site of an amino acid sequence shown in SEQ ID No.2. The amino acid sequence of the amine transaminase AcATA mutant is shown in SEQ ID No.2. The amino acid sequence of the amine transaminase AcATA mutant is shown in the description, wherein methionine at the 122 position is mutated into histidine, valine or phenylalanine. According to the invention, sites possibly influencing the catalytic activity are obtained through molecular docking, homologous modeling and other methods, and site-specific mutagenesis is carried out, so that the finally obtained aminotransferase AcATA mutant has high enzyme activity, and the enzyme activity is higher than 460U / g and is more than 4 times of that of a wild type; the catalyst can efficiently catalyze the sitagliptin intermediate precursor ketone 1-piperidine-4-(2, 4, 5-trifluorophenyl)-1, 3-dibutanone to synthesize the sitagliptin intermediate (R)-3-amino-1-piperidine-4-(2, 4, 5-trifluorophenyl)-1-butanone, and the 24-hour conversion rate is up to 90%.
Owner:ZHEJIANG UNIV OF TECH +2
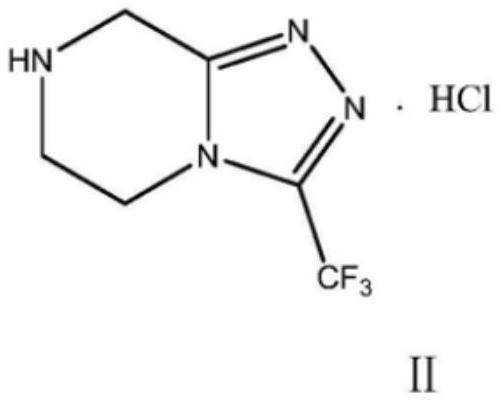
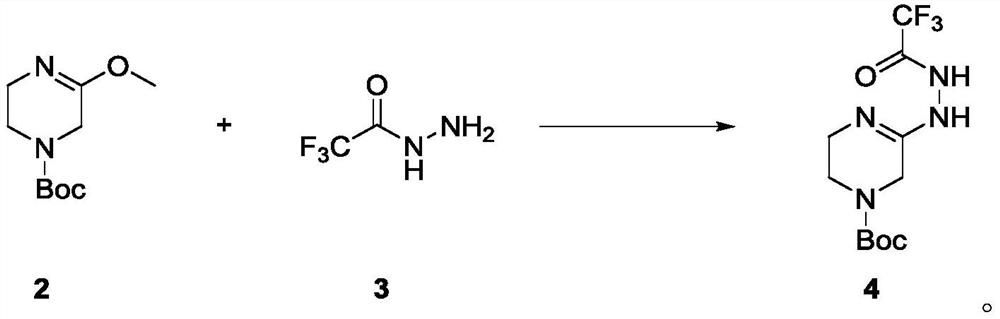
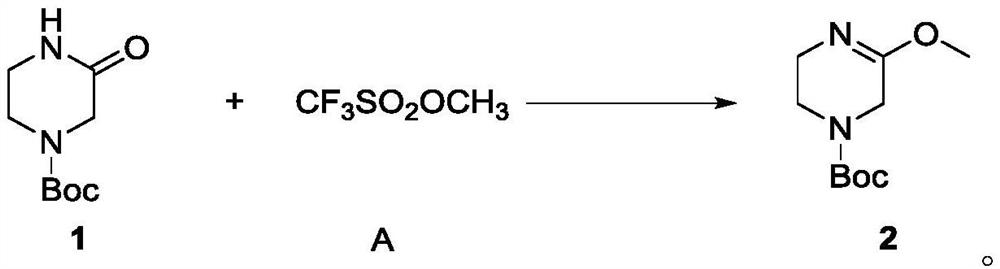
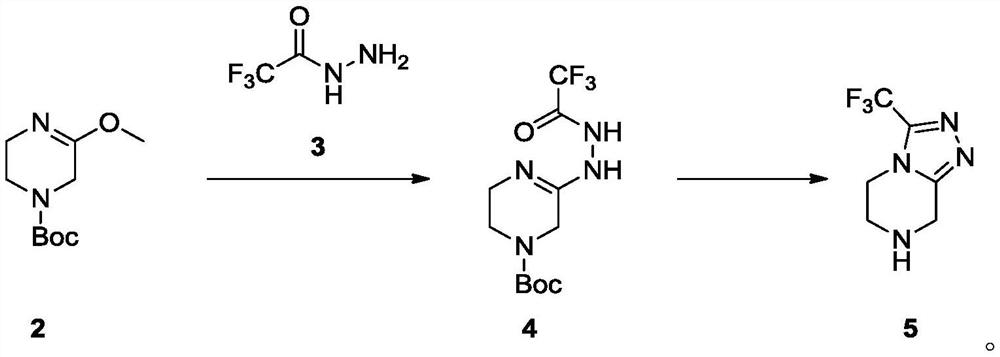
Preparation method of sitagliptin intermediate
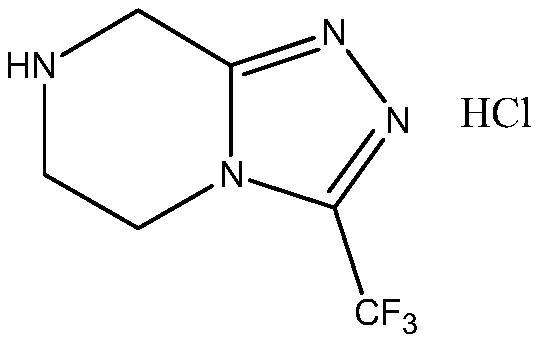
The invention relates to a preparation method of a sitagliptin intermediate, and belongs to the technical field of drug intermediate synthesis. In order to solve the problems of low product yield andpoor environmental protection property in the prior art, the invention provides a preparation method of a sitagliptin intermediate, and the method comprises the following steps: reacting 2-piperazinone with hydrazine hydrate in an alcohol solvent to generate piperazine hydrazone; in an acetonitrile or ether solvent, reacting the piperazine hydrazone with ethyl trifluoroacetate to obtain a corresponding intermediate N -[(2Z-) piperazine -2-subunit] trifluoroacethydrazide; and performing cyclization and salification reaction on the N -[(2Z-) piperazine -2-subunit] trifluoroacethydrazide in an alcohol solvent under the action of hydrochloric acid to obtain the corresponding sitagliptin intermediate 3-trifluoromethyl 5, 6, 7, 8-tetrahydro-1, 2, 4-triazole [4, 3-a] pyrazine hydrochloride. The reaction has the effects of high selectivity and high product yield.
Owner:江苏八巨药业有限公司
(R)-omega-transaminase mutant and application thereof
ActiveCN111411094AIncrease enzyme activityIncreased substrate toleranceBacteriaTransferasesArginineAcyl group
The invention discloses a (R)-omega-transaminase mutant and application thereof. The mutant is obtained by multi-point mutation of leucine at 182, arginine at 79, glutamine at 51, valine at 149, leucine at 235 and glycine at 216 in an amino acid sequence shown by SEQ ID NO.1. New (R)-omega-TA enzyme is screened through a gene mining technology in the invention; furthermore, molecular modificationis carried out through a protein engineering technology; a (R)-omega-TA mutant catalysis having high enzyme activity, high substrate tolerance and high stereoselectivity is obtained; the mutant can biologically catalyze precursor ketone analogue 1-(3-oxygen pyrrolidine-1-yl)-4-(2,4,5-trifluorophenyl)-1,3-butanedione synthesis sitagliptin intermediate (R)-1-[3-amino-4-(2,4,5-trifluorophenyl) butyryl] pyrrole-3-ketone; furthermore, the conversion rate is relatively high; the conversion rate can be up to 95.4%; and thus, the (R)-omega-transaminase mutant and application thereof in the invention have milestone meaning for breaking through a sitagliptin biocatalysis preparation technology.
Owner:ZHEJIANG UNIV OF TECH +2
Transaminase mutant and application thereof in preparation of sitagliptin intermediate
The invention discloses a transaminase mutant and application thereof in preparation of sitagliptin intermediate. The transaminase mutant is prepared by substituting tyrosine at locus 74 with proline,glutamic acid at locus 228 with aspartic acid, leucine at locus 254 with alanine and methionine at locus 290 with threonine in an amino acid sequence shown as SEQ ID NO: 2. According to the application of the transaminase mutant in the preparation of the sitagliptin intermediate, the sitagliptin intermediate is prepared by a method which takes wet thallus or pure enzyme obtained by fermenting andculturing engineering bacteria containing transaminase mutant encoding genes as a biocatalyst, as well as a sitagliptin intermediate precursor ketone or a prochiral carbonyl compound as a substrate.The total yield of the method is about 82%; and the e.e. value of the product can be up to 99%.
Owner:ZHEJIANG UNIV OF TECH +2
(R)-omega-transaminase mutant and application thereof in preparation of sitagliptin intermediate
ActiveCN111534494AIncrease enzyme activityIncreased substrate toleranceBacteriaTransferasesArginineThreonine
The invention discloses a (R)-omega-transaminase mutant and an application thereof in preparation of a sitagliptin intermediate. The mutant is obtained through multipoint mutation of arginine at the 77th site, leucine at the 181st site, arginine at the 130th site, tyrosine at the 139th site and threonine at the 273th site of an amino acid sequence shown as SEQ ID NO.1. According to the invention,a novel (R)-omega-TA recombinase is screened through a gene mining technology; molecular modification is carried out through a protein engineering technology; an (R)-omega-TA mutant catalyst with highenzyme activity, high substrate tolerance and high stereoselectivity is obtained; and the mutant can be used for asymmetric catalytic synthesis of the sitagliptin intermediate (R)-3-amino-1-(pyrrolidine-1-yl)-4-(2, 4, 5-trifluorophenyl) butan-1-one by taking a precursor ketone analogue 1-(pyrrolidine-1-yl)-4-(2, 4, 5-trifluorophenyl)-1, 3-butanedione as a substrate, and has higher conversion rate.
Owner:ZHEJIANG UNIV OF TECH +2
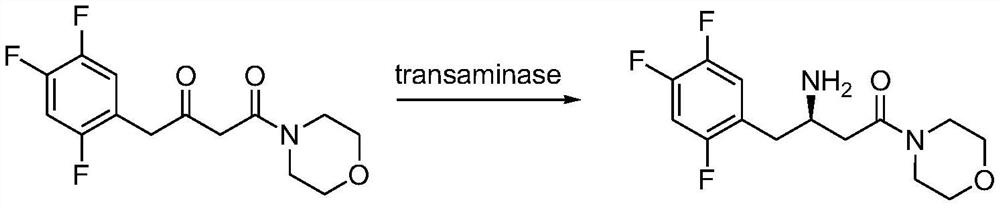
Transaminase mutant, immobilized transaminase and application of immobilized transaminase to preparation of sitagliptin
PendingCN113061594AImprove conversion rateHigh stereoselectivityBacteriaMicrobiological testing/measurementSitagliptinMorpholine
The invention provides application of immobilized transaminase in preparation of sitagliptin and / or (R)-3-amino-1-morpholine-4-(2,4,5-trifluorophenyl)-1-butanone. The immobilized transaminase comprises resin and a transaminase mutant, wherein the amino acid sequence of the transaminase mutant is shown as SEQ ID NO. 3 or SEQ ID NO. 7. The invention also provides the immobilized transaminase, a transaminase mutant and a preparation method and application of the immobilized transaminase and the transaminase mutant. The transaminase mutant is very high in enzyme activity when catalyzing a ketoamide substrate; the enzyme activity is still very high after the transaminase mutant is made into immobilized transaminase; and when the transaminase mutant is used for catalyzing a ketoamide substrate to produce sitagliptin or an intermediate thereof, a screened solvent reaction system is combined, and the immobilized transaminase is high in conversion rate, good in stereoselectivity, good in stability, higher in reusability and simpler to operate, so production cost is reduced, and industrial production is facilitated.
Owner:ABIOCHEM BIOTECH CO LTD
Intermediate for preparing Sitagliptin and preparation method and application of intermediate
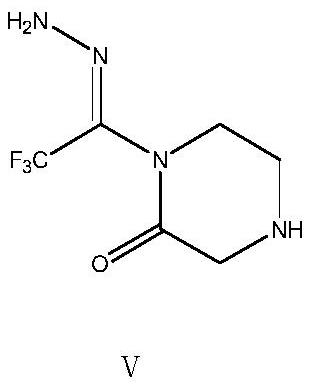
The invention relates to the technical filed of organic chemistry, in particular to an intermediate for preparing Sitagliptin and a preparation method and application of the intermediate. The structure of the intermediate is as shown in a formula A (Please see the formula A in the description.), wherein R adopts hydrogen or a formula as shown in the description.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
Recombinant R-type transaminase, mutant and application thereof
ActiveCN110904066AHigh activityIncreased substrate toleranceTransferasesGenetic engineeringNucleotideHigh activity
The invention relates to recombinant R-omega-transaminase, a mutant and an application of the recombinant R-omega-transaminase and the mutant in asymmetric synthesis of sitagliptin. According to the invention, novel R-omega-transaminase is screened and recombined with the existing high-efficiency transaminase; the amino acid sequence of the obtained recombinant is shown as SEQ ID: 15, and the mutant thereof is obtained by carrying out single-site mutation or multi-site mutation on one or more of 71, 135 and 292 amino acid sequences shown in SEQ ID: 15; the optimal amino acid sequence of the R-omega-transaminase mutant strain is shown as SEQ ID: 17; and the corresponding nucleotide sequence is shown as SEQ ID: 18. The invention provides the R-omega-TA mutant with higher activity (582.4U / g) and stereoselectivity (e.e.value 99.9%). A 500mM substrate can be catalyzed to be converted into sitagliptin, and the conversion rate is as high as 99%. The mutant has an important significancefor improving the sitagliptin biocatalytic preparation technology.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing sitagliptin intermediate via asymmetrical reduction method
ActiveUS20170305822A1Atom utilization is highShort stepsCarbamic acid derivatives preparationOrganic compound preparationPtru catalystPhosphine
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Preparation method of sitagliptin intermediate
InactiveCN112979564AReduce pollutionRaw materials are cheap and easy to getOrganic chemistrySitagliptinMedicinal chemistry
The invention discloses a preparation method of a sitagliptin intermediate. The preparation method of the sitagliptin intermediate comprises the following step: in a solvent, carrying out a reaction as shown in the specification on a compound as shown in a formula 2 and a compound as shown in a formula 3 to obtain a compound as shown in a formula 4. The preparation method is high in yield, avoids the use of explosive reagents and phosphorus oxychloride, and is suitable for industrial production.
Owner:SHANGHAI SUNTECH PHARMA
Preparation method of sitagliptin intermediate
ActiveCN113481254AHigh recovery rateEasy to separate and purifyChemical recyclingFermentationPyrazineOrganosolv
The invention discloses a preparation method of a sitagliptin intermediate and the sitagliptin intermediate prepared by the preparation method, the preparation method comprises the step that in the presence of an organic solvent with a boiling point not higher than 110 DEG C at a standard atmospheric pressure, carrying out transamino contact between transaminase and a substrate sitagliptin precursor ketone (2Z)-4-oxo-4-[3-(trifluoromethyl)-5, 6-dihydro-[1, 2, 4]triazolo[4, 3-a]pyrazine-7-(8H)-yl]-1-(2, 4, 5-trifluorophenyl) butyl-2-one to perform enzyme catalysis reaction so as to prepare the sitagliptin intermediate (3R)-3-amino-1-[3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-1, 2, 4-triazolo [4, 3-a] pyrazine-7-yl]-4-(2, 4, 5-trifluorophenyl) butyl-1-one, a mixed solution obtained by mixing a low-boiling-point organic solvent and the substrate is added into a premixing system containing the transaminase in a fed-batch mode, ketoreductase and a coenzyme regeneration system in a specific proportion are added into the premixing system, the substrate conversion rate is increased, and the substrate conversion rate can reach 99%.
Owner:台州酶易生物技术有限公司
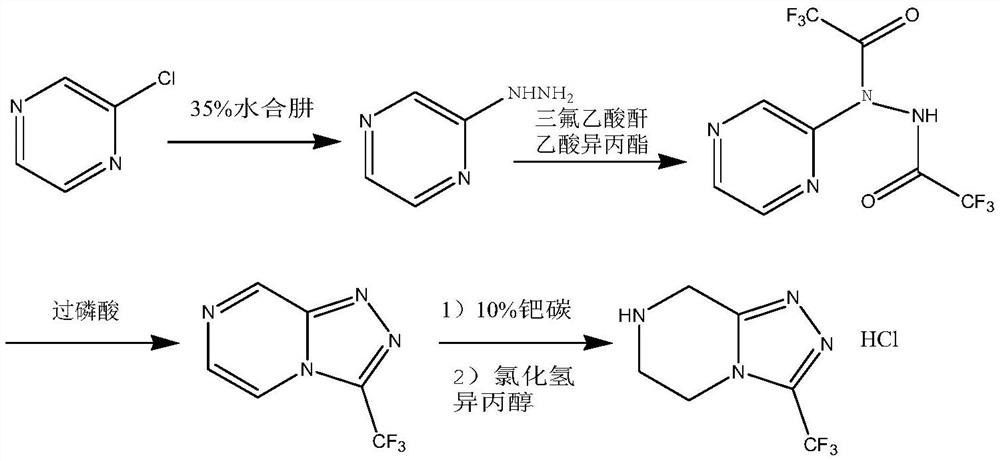
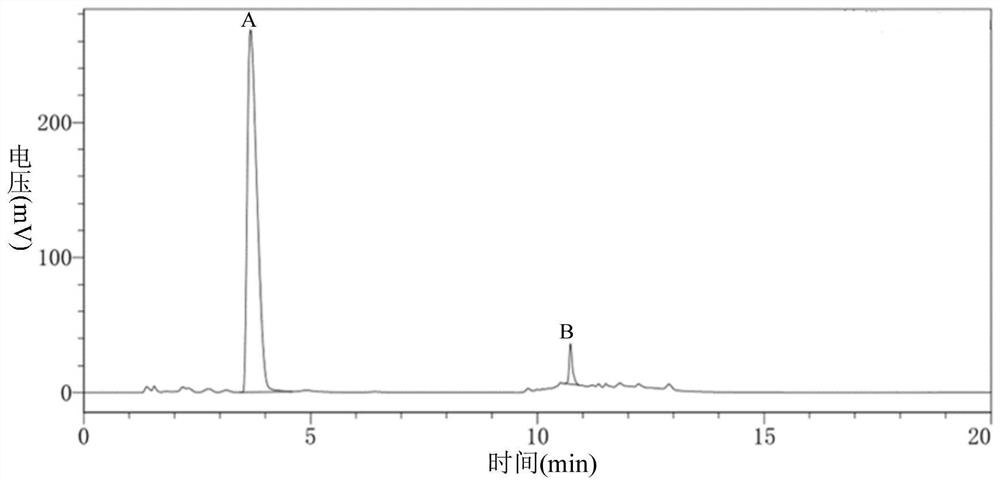
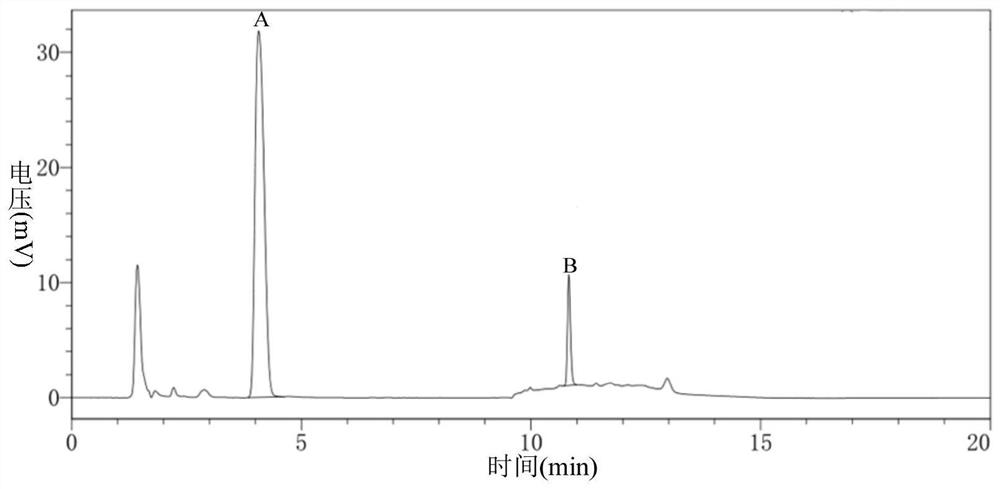
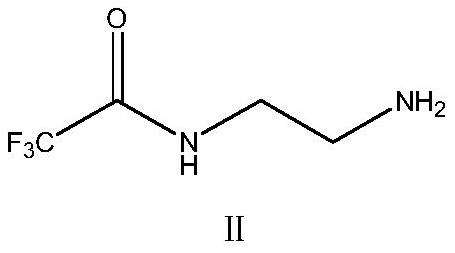
Green synthesis method of sitagliptin intermediate
ActiveCN113527312AHigh yieldImprove the quality of purityOrganic chemistryBulk chemical productionEthyl acetateEther solvent
The invention relates to a green synthesis method of a sitagliptin intermediate, and belongs to the technical field of drug intermediate synthesis. In order to solve the problems of serious pollution and instability in the prior art, the invention provides a green synthesis method of a sitagliptin intermediate, which comprises the following steps: reacting ethyl trifluoroacetate with ethylene diamine in an ether solvent to generate 2-trifluoroacetamido ethyl amine; in the presence of an acid-binding agent, carrying out condensation reaction on 2-trifluoroacetamido ethyl amine and halogenated ethyl acetate at the temperature of 45-65 DEG C to generate an intermediate, then heating to 90-110 DEG C, and carrying out cyclization reaction to generate N-trifluoroacetyl piperazinone; carrying out reaction on N-trifluoroacetyl piperazinone and hydrazine hydrate to generate 1-trifluoroacetyl hydrazino-2-piperazinone; and reacting the intermediate with hydrochloric acid to carry out internal ring formation and salt formation reaction to obtain the product sitagliptin intermediate. According to the invention, the reaction has the advantages of high product yield and purity on the whole, and is environment-friendly.
Owner:江苏八巨药业有限公司
Medicinal composition for treating diabetes and obesity
The invention belongs to the technical field of medicinal preparations and particularly relates to a medicinal composition for treating diabetes and obesity and preparation and use thereof. The invention is characterized in that the active ingredient of a single dose of medicine comprises 10 to 90 milligrams of Sitagliptin or salt thereof based on Sitagliptin and 5 to 50 milligrams of Simvastatin or Lovastatin, preferably 25 to 50 milligrams of Sitagliptin or salt thereof and 5 to 25 milligrams of Simvastatin or Lovastatin. The medicinal composition can be prepared into any formulation acceptable in pharmaceutics, preferably oral solid preparation.
Owner:郑飞雄
Preparation method of sitagliptin
ActiveCN102627648BLow costHigh yieldOrganic chemistryBulk chemical productionPhenylacetic acidMethyl palmoxirate
The invention provides a preparation method of sitagliptin. The preparation method comprises the following steps of: performing condensation reaction on hydrochloride of 3-trifluoromethyl-[1,2,4] triazol [4,3-a] piperazine serving as a starting raw material and methyl malonyl chloride under a normal temperature condition; reacting an obtained product with 2,4,5-trifluorophenylacetic acid under an alkaline condition and then performing condensation reaction with (S)-phenylglycinamide under normal temperature condition to obtain a product; reducing the obtained product through a reducing agent; removing an ester group through heating reflux; and reacting with a hydrogenation reducing reagent to obtain the sitagliptin. The preparation method has the advantages of low cost, high yield, easiness in operation, all used reagents of conventional reagents, simple post-treatment and convenience for industrial production.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Transaminase and application thereof in preparation of sitagliptin
The invention discloses transaminase and application thereof in preparation of sitagliptin. The amino acid sequence of the transaminase is shown as SEQ ID NO: 1, or the amino acid sequence of the transaminase is at least 80%, at least 85%, at least 90%, at least 95%, at least 96%, at least 97%, at least 98% or at least 99% similar to the SEQ ID NO: 1. The transaminase can asymmetrically catalyze sitagliptin precursor ketone (2Z)-4-oxo-4-[3- (trifluoromethyl)-5, 6-dihydro-[1, 2, 4] triazolo [4, 3-a] pyrazine-7-(8H)-yl]-1-(2, 4, 5-trifluorophenyl) butyl-2-ketone to generate sitagliptin intermediate (3R)-3-amino-1-[3-(trifluoromethyl)-5, 6, 7, 8-tetrahydro-1, 2, 4-triazolo [4, 3-a] pyrazine-7-yl]-4- (2, 4, 5-trifluorophenyl) butyl-1-ketone, the enzyme activity of the transaminase can reach 1100U / g, and when an enzymatic reaction is carried out for 2h, 50 microliters of transaminase liquid can make the substrate conversion rate reach 55%. Compared with existing transaminase for catalyzing the same reaction, the transaminase has the advantages of high activity, ideal stability and good organic solvent tolerance.
Owner:台州酶易生物技术有限公司
Process for the preparation of triazole and salt thereof
ActiveUS20180230151A1Improve productivityAvoid the needOrganic active ingredientsOrganic chemistryCombinatorial chemistryMedicinal chemistry
An improved process for the preparation of Triazole and salts thereof, a key intermediate for the synthesis of Sitagliptine is disclosed.
Owner:F I S FAB ILTALIANA SINTETICI SPA
Recycling method of acetyl Meldrum's acid derivative degradation waste
PendingCN113045448ARealize resource reuseReduce economic lossOrganic compound preparationCarboxylic acid esters preparationSitagliptinAcid derivative
The invention provides a recycling method of acetyl Meldrum's acid derivative degradation waste, and belongs to the technical field of resource reutilization. According to the method disclosed by the invention, acetyl Meldrum's acid derivative degradation waste is subjected to preheating treatment, alcoholysis reaction, ammonolysis reaction, acetylation reaction and purification treatment, the main components in theacetyl Meldrum's acid derivative degradation waste are converted, and a recovery product is finally obtained and can be used as an important intermediate in a sitagliptin production process, so that waste resource reutilization in the production process of the sitagliptin bulk drug is realized, economic loss caused by degradation of the acetyl Meldrum's acid derivative in the production process of the sitagliptin bulk drug is reduced, the problem of environmental pollution caused by degradation of the acetyl Meldrum's acid derivative in the production process of the sitagliptin bulk drug is eliminated, and the sustainable development of a sitagliptin raw material medicine green pharmaceutical process is promoted.
Owner:TAIZHOU BIOMEDICAL & CHEM IND RES INST CO LTD
Sitagliptin impurity, and preparation method and detection method thereof
InactiveCN113461691AQuality improvementControl detectionOrganic chemistryComponent separationSitagliptinDrug utilisation
The invention relates to a sitagliptin impurity, and a preparation method and a detection method thereof. The new impurity found by the invention can provide standard reference for quality control of sitagliptin and safety detection of clinical medication, so that the safety and reliability of clinical medication are ensured. Besides, the preparation method of the impurity compound provided by the invention is simple to operate and mild in reaction condition, the impurity compound with high purity and high yield can be obtained, and the preparation method is used for further researching the property of the impurity and providing technical support for controlling the content of the sitagliptin compound and solving the problems of impurity limit of sitagliptin and safety of related products; and the detection method of the sitagliptin impurity can carry out targeted qualitative detection on the impurity, is high in detection precision, and is beneficial to providing a technical means for detection of the impurity in the sitagliptin, so that the quality of the sitagliptin is controlled.
Owner:ZHEJIANG MENOVO PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com