Aspartase mutant and applications thereof
An aspartase and mutant technology, applied in application, enzyme, lyase and other directions, can solve the problem of low catalytic activity, and achieve the effects of high stereoselectivity, high conversion rate and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Construction of AspB (aspartase, aspartase) mutant library
[0059] The AspB enzyme derived from Bacillus sp.YM55-1 was retrieved from NCBI, PDB No. 1J3U_A, the amino acid sequence is SEQ ID NO.2, the gene sequence is SEQ ID NO.1, targeting the 187th and 321st positions of SEQ ID NO.2 Position, 324, 326, 358 mutation library construction designed primer sequences are shown in Table 3:
[0060] Table 3 Primer sequence list
[0061]
[0062] Wherein, N represents any nucleotide in A, G, C, T, M represents A or C, and K represents G or T; it is selected according to the coding nucleotide of the amino acid to be mutated into at the site , such as NNK in the C187NNK forward primer can represent AAG (lysine), AAT (aspartic acid), AGG (arginine) or AGT (serine), etc., and the nucleotides corresponding to specific amino acids can be found in the table 2.
[0063] The gene AspB was synthesized according to the sequence of SEQ ID NO.1 in the sequence listing. The ...
Embodiment 2
[0071] Embodiment 2 High-throughput screening mutant library
[0072] Screen according to the following experimental steps
[0073] The first step of primary screening is carried out by using the screening method of nylon membrane transfer. When the transformant grows on the plate in Example 1, use the sterilized nylon membrane to blot the colony on the plate, and then use 500 μL of the colony on the membrane to contain 80 μg / mL ampicillin, 0.1mM IPTG, and 0.01mM MgCl 2 Wash the colonies with the TB medium, transfer the washed bacterial solution to a 96-well plate for induction, and induce overnight at 200 rpm at 25°C, then centrifuge at 4000 rpm for 20 minutes to collect the bacteria, discard the culture medium, and finally add 60 μL bugbuster to lyse, and lyse at 30°C for 2 hours. The obtained enzyme lysate was reacted according to the reaction system in Table 6, and reacted at 45° C. and 220 rpm for 3 days, and the reaction effect was detected by HPLC.
[0074] Table 6 Pr...
Embodiment 3
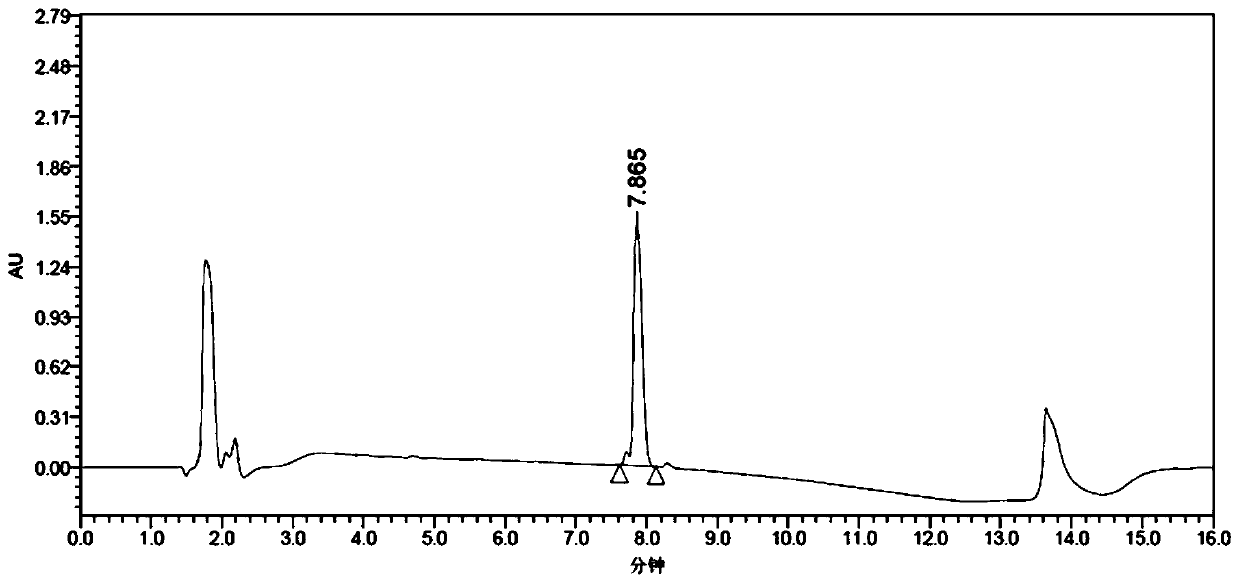
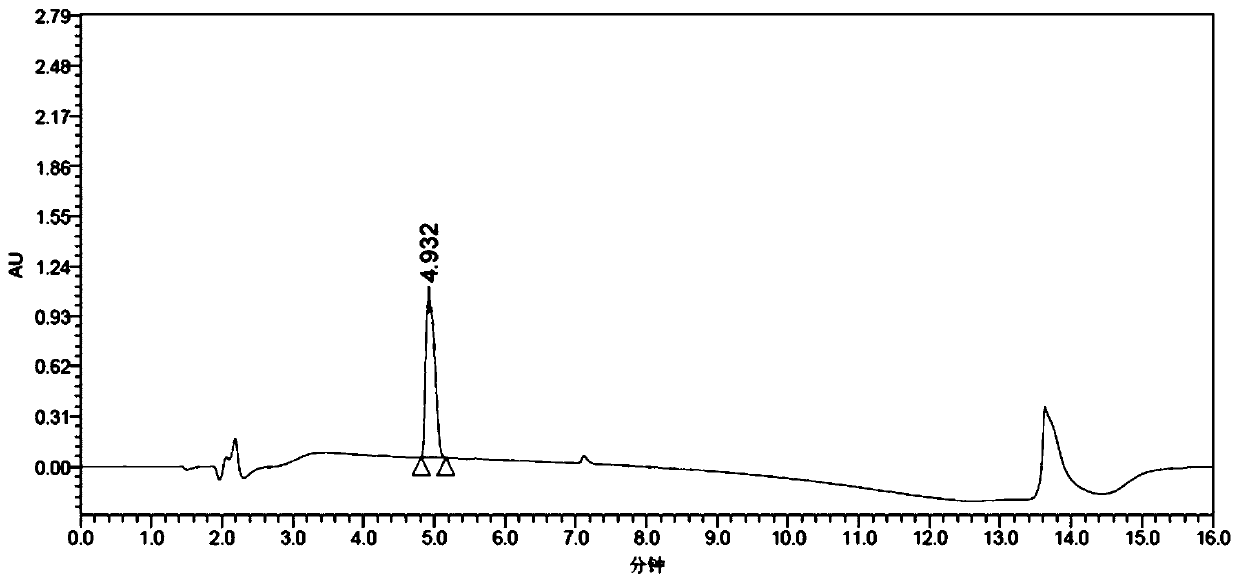
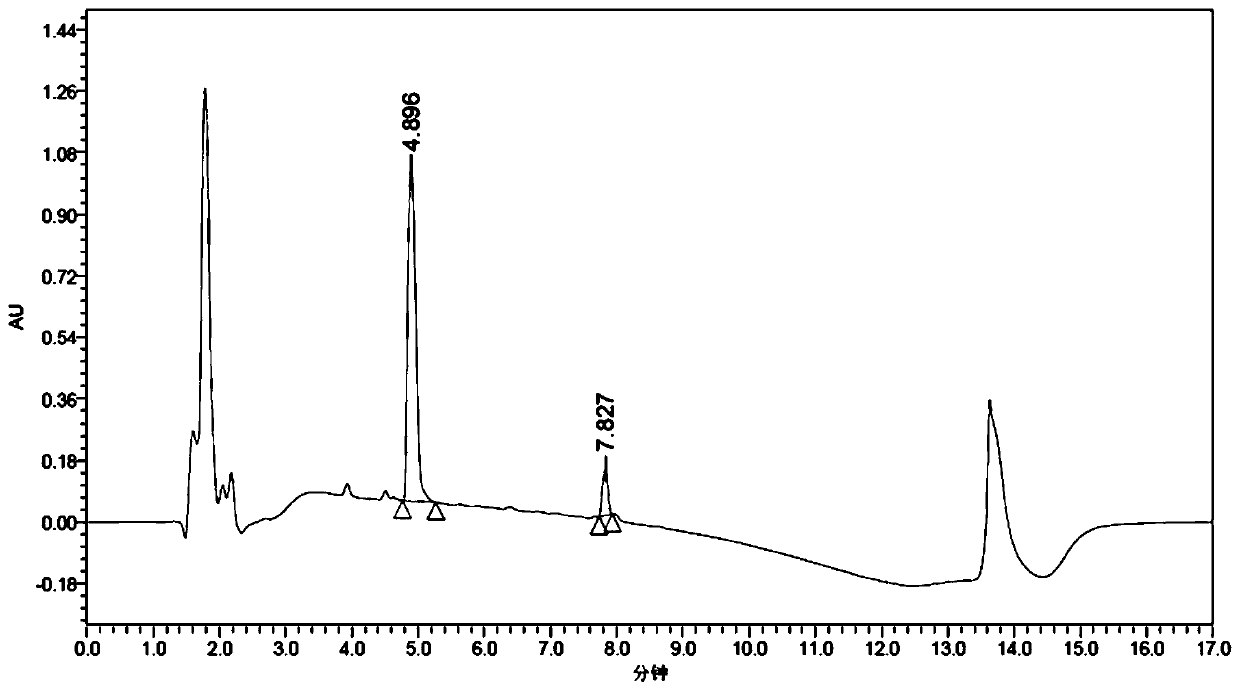
[0089] Example 3 Application of AspB mutant in the preparation of (R)-3-amino-4-(2,4,5-trifluorophenyl)-butyric acid
[0090]
[0091] Accurately weigh 60.6 mg of Tris base, 216 mg of (E)-4-(2,4,5-trifluorophenyl)but-2-enoic acid, 0.5 g of NH 4Cl, add to 7mL water containing 5% DMSO (dimethyl sulfoxide), adjust the pH to 8.5 with ammonia water, finally add 1g of the fungus slime prepared according to the method in Example 2 to the reaction system, and finally make up 2-3mL of water to the final volume 10mL. At 45°C, 220rpm, overnight 12h reaction, HPLC analysis conversion rate (after this embodiment does not directly detect the ee of product (R)-3-amino-4-(2,4,5-trifluorophenyl)-butyric acid value, but the product is continued to react to obtain (3R)-N-tert-butoxycarbonyl-3-amino-4-(2,4,5-trifluorophenyl)-butyric acid after detection ee value), after Add 218 mg of di-tert-butyl dicarbonate (Boc anhydride) and react for 48 hours, take the reaction solution to measure the e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com