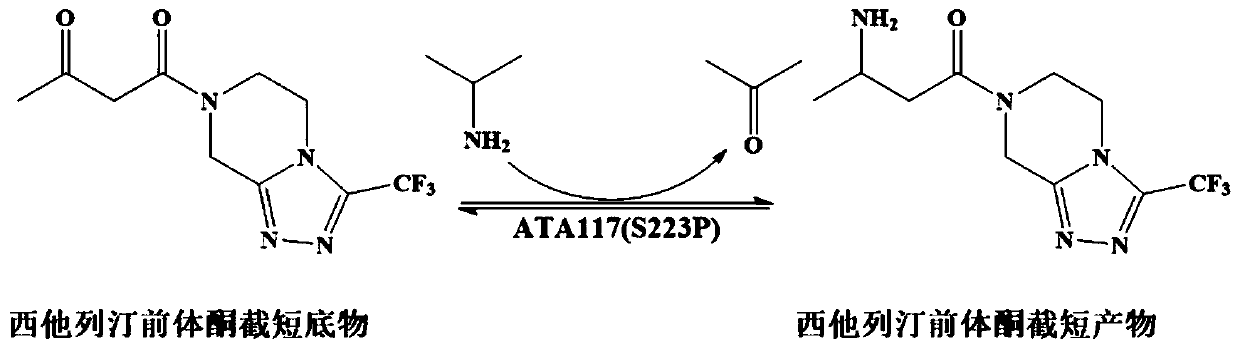
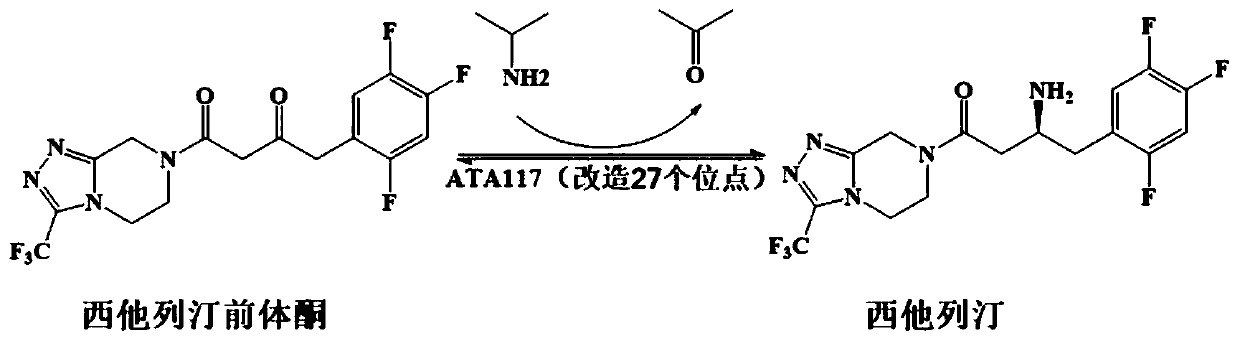
Recombinant R-type transaminase, mutant and application thereof
A transaminase and mutant technology, which is applied in the field of recombinant R-ω-transaminase and mutants, can solve the problems of harsh reaction conditions, high cost of separation and high residual rate of metal ions, and achieves the effect of high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0028] Example 1: Screening of novel ω-TA, determination of stereoselectivity and precise determination of enzyme activity
[0029] 1. Enzyme source and gene synthesis
[0030] Using gene mining technology, three new enzymes were obtained from NCBI database gene mining, respectively from Mycolicibacteriumgoodii (MgTA, Genbank number WP_049743179.1), Amine transaminaseHFO (HFO, Genbank number AIN35005.1) and Mesorhizobium japonicum (MjTA, Genbank number WP_010910285 .1). Codon optimization was carried out according to the codon preference of E.coli, and the nucleotide sequences of the three enzymes were synthesized by the method of whole gene synthesis, which are respectively shown in SEQ ID NO.2, 4 and 6; the amino acid sequences of the encoded enzymes were respectively As shown in SEQ ID NO.1, 3 and 5. Add 6×his-tag tags at the end of the nucleotide sequence, add restriction sites Xho I and Nco I at both ends, clone the gene into the Xho I and NcoI sites corresponding to pE...
Embodiment 3
[0045] Example 3: Recombination and screening of enzymes
[0046] The research group applied for the high-efficiency R-selective Gibberellazeae TA mutant (hereinafter referred to as GzTA) in the patent CN201910871261.3 7 ), the activity to sitagliptin precursor ketone is 210.3U / g, can catalyze the conversion of 200mM precursor ketone into sitagliptin, and the conversion rate is 82.6%. (GzTA 7 The nucleotide sequence of the enzyme is shown in SEQ ID NO.8, and the amino acid sequence of the encoded enzyme is shown in SEQ ID NO.7). In the present invention, DNA recombination is carried out respectively with MjTA and MgTA to obtain new mutants.
[0047] 1. Clone GzTA 7 , MjTA and MgTA catalytic regions
[0048] (1) GzTA synthesized as needed 7 Design primers for the nucleotide sequence fragments, and use rapid PCR technology to recombine the vector pET28b / GzTA 7 As a template, synthesize GzTA 7 The N-terminal catalytic region (referred to as A 1 ) DNA fragment, the primers...
Embodiment 4
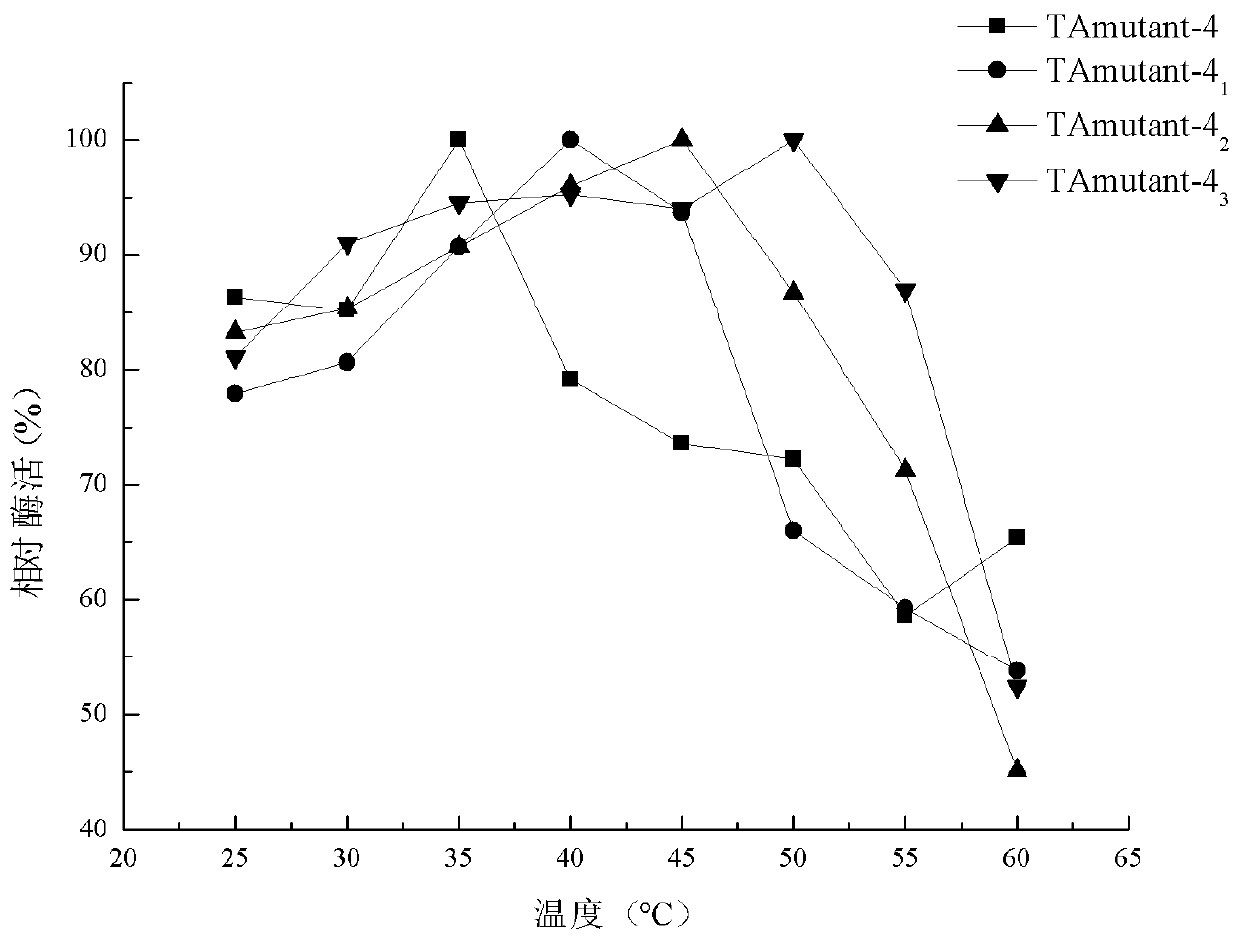
[0112] Example 4: Construction and screening of TAmutant-4 single point mutants
[0113] 1. Construction of mutants
[0114] The R-ω-TA screened in the mutant library was subjected to single-point mutation, and primers for site-directed mutation were designed according to the amino acid sequence of TAmutant-4 (SEQ ID NO.15), and the recombinant vector pET28b / TAmutant- 4 is a template, and a single point mutation is introduced at position 71 of the amino acid sequence of TAmutant-4, and the primers are:
[0115] Forward primer 71H: GTTCTTCCGT NNK GACGACCAC (the underline is the mutated base)
[0116] Reverse primer 71H: GTGGTCGTC MNN CGGAAGAAC (the underline is the mutant base)
[0117] PCR reaction system: 2×Phanta Max Buffer (containing Mg 2+ )25μL, dNTPs10mM, forward primer 71H 2μL, reverse primer 71H 2μL, template DNA 1μL, Phanta Max Super-Fidelity DNA Polymerase 50U, add ddH 2 0 to 50 μL.
[0118] The PCR amplification conditions were 95°C for 3min; (95°C for 15s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com