Preparation method of sitagliptin intermediate
A volume ratio, compound technology, applied in the field of preparation of sitagliptin intermediates, can solve the problems of low yield, unsuitable for industrial production, environmental pollution, etc., and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
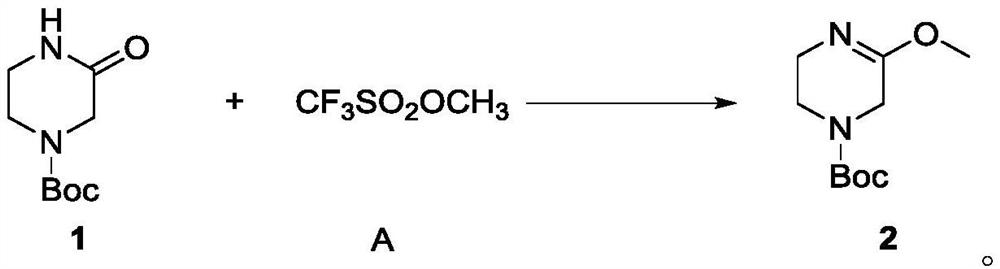
[0070] In a 100ml single-neck bottle, add Intermediate 1 (2g, 10mmol) and dissolve it in dichloromethane (40ml), add methyl trifluoromethanesulfonate (3.28g, 2eq, 20mmol) dropwise under an ice-water bath, and slowly add The temperature was slowly raised to 40°C, and the reaction was carried out at this temperature for two hours, and the completion of the reaction was monitored by TLC. The reaction was cooled to room temperature, potassium carbonate (3.06g, 2.2eq, 22mmol) aqueous solution (20ml) was added, stirred for half an hour, and the organic phase was collected by separation. The aqueous phase was extracted three times with dichloromethane (30ml*3), the organic phases were combined and concentrated to obtain a colorless oily liquid 2 (1.8g, yield 84.1%), 1 H-NMR (400MHz, DMSO-d6): δ3.81 (s, 2H), 3.58 (s, 3H), 3.41-3.39 (m, J=8.0Hz, 2H), 3.31-3.26 (m, J=10.0 Hz, 2H), 1.41(s, 9H).
Embodiment 2
[0072]
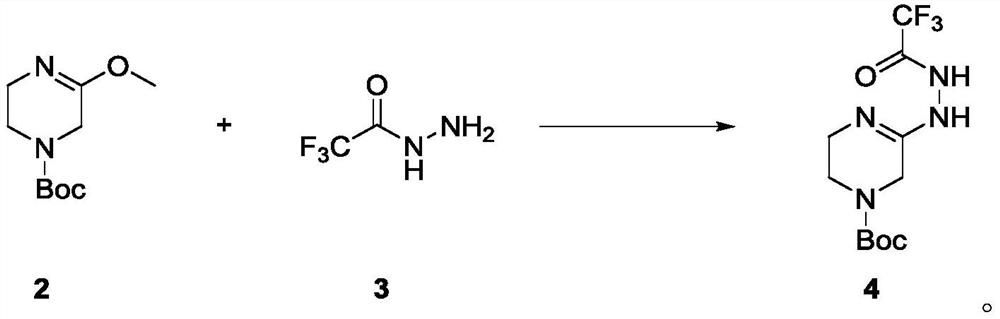
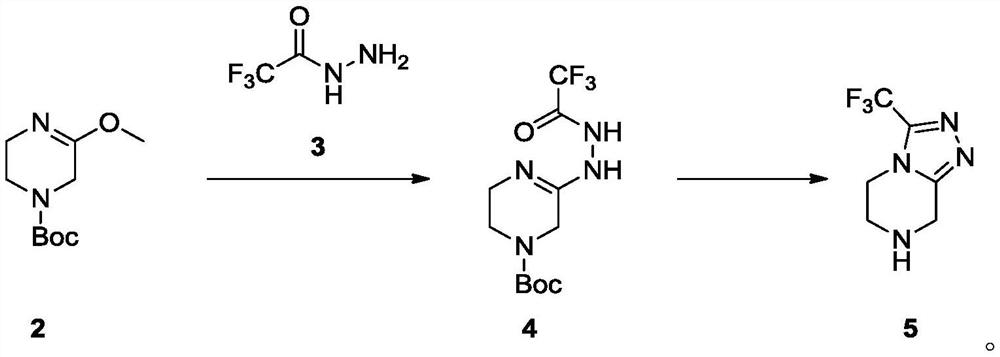
[0073] In a 100ml single-necked bottle, add Intermediate 2 (1g, 4.67mmol) dissolved in acetonitrile (20ml), add trifluoroacetonitrile (658mg, 1.1eq, 5.14mmol) in acetonitrile (20ml) dropwise at room temperature, after the addition is complete The reaction was carried out at room temperature for 5 h, and the reaction was completed by TLC monitoring. The reaction solution was concentrated, separated and purified by normal phase silica gel column (methanol: dichloromethane=0-5% as mobile phase) to obtain colorless oily liquid 4 (1.2g, yield 82.8%), LCMS[M+Na] - :333.7. 1 H-NMR (400MHz, DMSO-d6): δ4.77(s, 2H), 4.18-4.15(t, J=12.0Hz, 2H), 3.84-3.81(t, J=12.0Hz, 2H), 1.44( s, 3H).
Embodiment 3
[0075]
[0076] In a 100ml single-neck bottle, intermediate 4 (120mg, 0.39mmol) was dissolved in methanol (10ml), the reaction solution was heated to 55°C, and concentrated hydrochloric acid (0.05ml, 1eq, 0.39mmol) was slowly added dropwise at this temperature. , After the dropwise addition, the reaction was carried out at this temperature for 2h, and a solid was gradually precipitated, and the TLC monitoring reaction was completed. The reaction solution was cooled to room temperature, stirred at room temperature for 0.5 h, added MTBE (30 ml), a solid was precipitated, filtered, and the filter cake was washed with ethanol: MTBE=1:3 (30 ml) to obtain a white solid, which was vacuum-dried at 45 °C to obtain the key intermediate The hydrochloride salt of 5 (78 mg, 88.1% yield), LCMS [M+H] - : 192.8. 1 H-NMR (400 MHz, DMSO-d6): δ10.50(s, 2H), 4.61(s, 2H), 4.46-4.43(t, J=12.0Hz, 2H), 3.65-3.62(t, J= 12.0Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com