Process for the preparation of triazole and salt thereof
a triazole and salt technology, applied in the field of triazole and salt thereof, can solve the problems of long cycle time, complex recovery of solvents, and relatively low productivity of triazole, and achieve the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
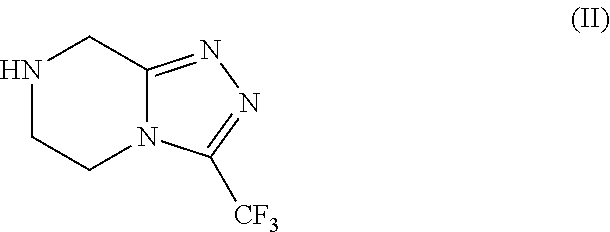
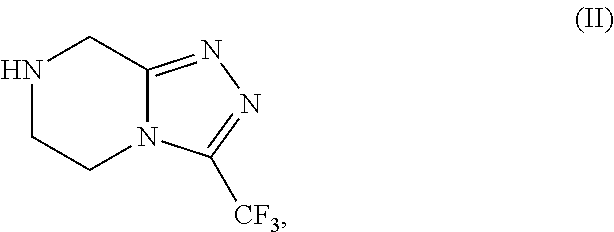
[0088]Preparation of Triazole of formula (II) as HCl salt in 5 volumes of ethanol with the presence catalytic amount of water.
[0089]Into a three necked round bottom flask, under nitrogen atmosphere, 250 g of Amidine of formula (III), 1000 mL of denatured ethanol (4V) and 12.5 mL of water (0.05V). The obtained suspension is heated at temperature 60° C. and stirring at the same temperature at least 15 min. Then a solution of 45 g HCl (gas) in 250 mL denatured ethanol (1V) was dosed in 20 min., keeping the temperature at temperature range from 55° C. to 60° C. The resulting mixture was stirred for 1 hour at the same temperature. The slurry was cooled at room temperature and was stirred for least 30 min. at the same temperature. The obtained slurry was filtered and the solid was washed with 250 mL of denatured ethanol. Drying the solid under vacuum at T=45° C., 246.25 g of Triazole of formula (II) as HCl salt were obtained with 90.53% of molar yield.
example 2
[0090]Preparation of Triazole of formula (II) as HCl salt in 1.7 volumes of ethanol.
[0091]Into a three necked round bottom flask, under nitrogen atmosphere, 500 g of Amidine of formula (III), 450 mL of denatured ethanol (0.9V). The obtained suspension is heated at temperature 60° C. and stirring at the same temperature at least 15 min. Then a solution of 90 g HCl (gas) in 315 mL denatured ethanol (0.8V) was dosed in 20 min., keeping the temperature at temperature range from 55° C. to 60° C. The resulting mixture was stirred for 1 hour at the same temperature. The slurry was cooled at room temperature and was stirred for least 30 min. at the same temperature. The obtained slurry was filtered and the solid was washed with 500 mL of denatured ethanol. Drying the solid under vacuum at T=45° C., 493.5 g of Triazole of formula (II) as HCl salt were obtained with 90.71% of molar yield.
example 3
[0092]Preparation of Triazole of formula (II) as HCl salt in 1.7 volumes of ethanol.
[0093]Into a three necked round bottom flask, under nitrogen atmosphere, 180 g of Amidine of formula (III) and 180 mL of denatured ethanol (1V). The obtained suspension is heated at temperature 60° C. and stirring at the same temperature at least 15 min. 122,1 g of a solution of HCl (gas) on denatured ethanol (0.7V) were dosed for 20 min., keeping the temperature at temperature range from 60° C. to 70° C. The resulting mixture was heated a reflux (T=79° C.) for 30 min. The slurry was cooled at room temperature and was stirred for least 30 min. at the same temperature. The obtained slurry was filtered washing the solid with 180 mL of denatured ethanol. Drying the solid under vacuum at T=50° C., 175,7 g of Triazole of formula (II) as HCl salt were obtained with 89,94% of molar yield and HPLC purity of 99.8% A / A%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com