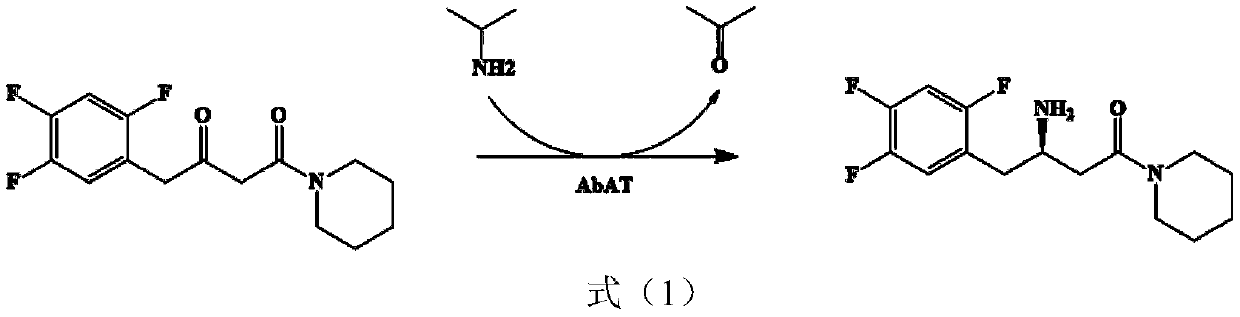
Amine transaminase AcATA mutant and application thereof in preparation of sitagliptin intermediate
A technology of amine aminotransferase and sitagliptin, which is applied in the application field of amine aminotransferase AcATA mutant and the preparation of sitagliptin intermediates, can solve the problems of narrow substrate spectrum, limited application scope and the like, and achieves the improvement of the overall conversion rate , the effect of shortened response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Homology modeling and substrate docking of wild-type sequences
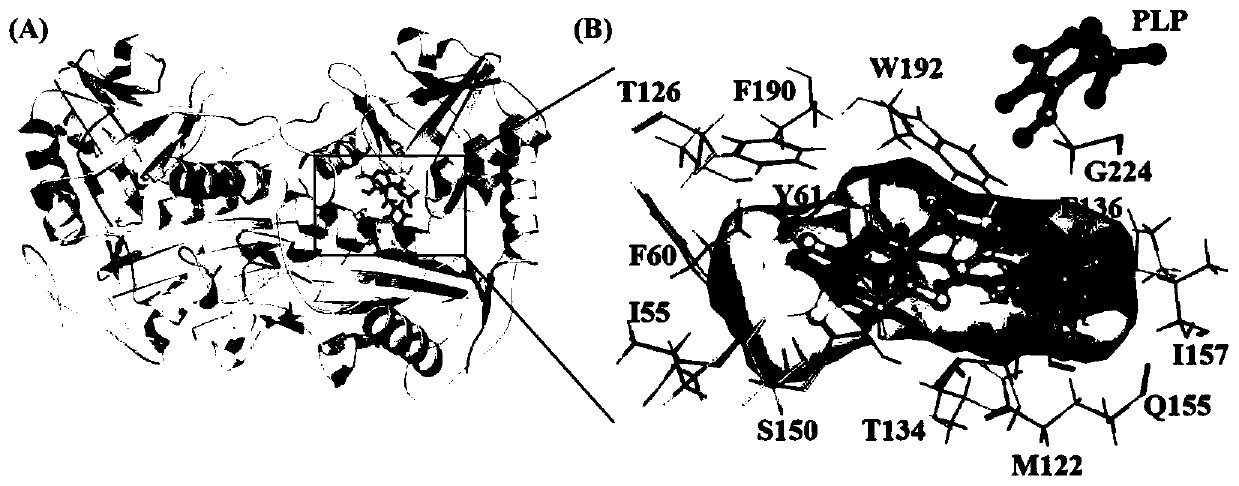
[0040] Using the crystal structure of AcATA (PDB-ID: 3WWJ) as a template, SWISS-MODEL was used for homology modeling, and the AcATA model structure was superimposed on the wild-type model structure using a proprietary algorithm based on the comparison of secondary structure elements, and passed through FoldX The software is optimized. The optimized homology model was docked with the substrate through Autodock software, and the docking result with the lowest energy was selected for key site analysis ( figure 1 ).
Embodiment 2
[0041] Example 2: Alanine scanning mutagenesis and recombinant E. coli cell culture
[0042] All the amino acid positions obtained after docking the substrate of Example 1 were scanned for alanine using the QuikChange site-directed mutagenesis kit and Phanta Max ultra-fidelity DNA polymerase (Vazyme, Nanjing, China).
[0043] Some primers are:
[0044] Primer 1: M122 PrGAGCTGCGTGAAGCGGCGGTAACTGTTACCATC
[0045] M122 Pf GATGGTAACAGTTACCGCCGCTTCACGCAGCTC
[0046] Primer 2: G224PrCTGGCGGAAGGTCCGGCTTTCAACGTAGTAGTG
[0047] G224 Pf CACTACTACGTTGAAAGCCGGACCTTCCGCCAG
[0048] Primer 3: W192 Pr GTGAAAAACTTCCAGGCGGGTGATCTGATTCGT
[0049] W192 Pf ACGAATCAGATCACCCGCCTGGAAGTTTTTCAC
[0050] Primer 4: T126 Pr GCGATGGTAACTGTTGCCATCACTCGTGGTTAC
[0051] T126 Pf GTAACCACGAGTGATGGCAACAGTTACCATCGC
[0052] Primer 5: S150 Pr CCTCAGGTGTACATGGCTGCATGTCCGTACCAG
[0053] S150 Pf CTGGTACGGACATGCAGCCATGTACACCTGAGG
[0054] Primer 6: F60 PrATCTTCGACCAGGGCGCTTATACTTCCGATGCG
[0055] F60 Pf CGC...
Embodiment 3
[0063] Example 3: Induced expression of amine transaminase AcATA
[0064] The ω-transaminase mutant containing the complete open reading frame was cultivated according to the method of obtaining wet cells in Example 2, and the obtained wet cells could be directly used as a biocatalyst for subsequent enzyme activity determination or protein purification.
[0065] The preparation method of wet thallus (that is: genetically engineered bacteria) is: inoculate the recombinant Escherichia coli containing the ω-transaminase mutant coding gene to the LB liquid medium containing 50 μg / ml ampicillin, cultivate 8- 10h, obtain the seed liquid; then inoculate the seed liquid with a volume concentration of 1-2% inoculum into fresh LB liquid medium containing 50 μg / ml ampicillin resistance, and cultivate it at 37°C and 150 rpm until the cell OD 600 After reaching 0.6-0.8, add IPTG with a final concentration of 0.1mM, induce culture at 28°C for 12h, centrifuge at 8000rpm at 4°C for 10min, dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com