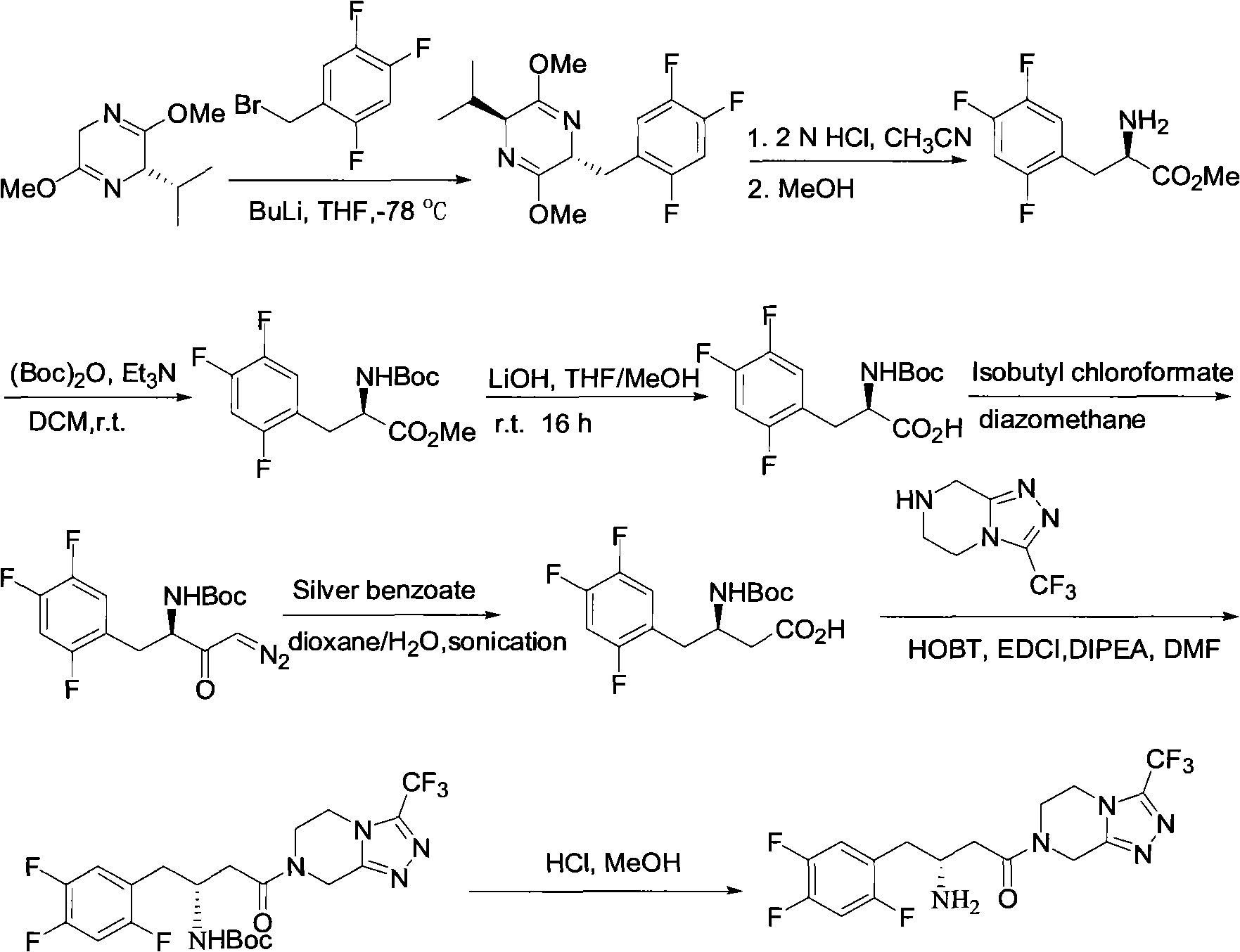
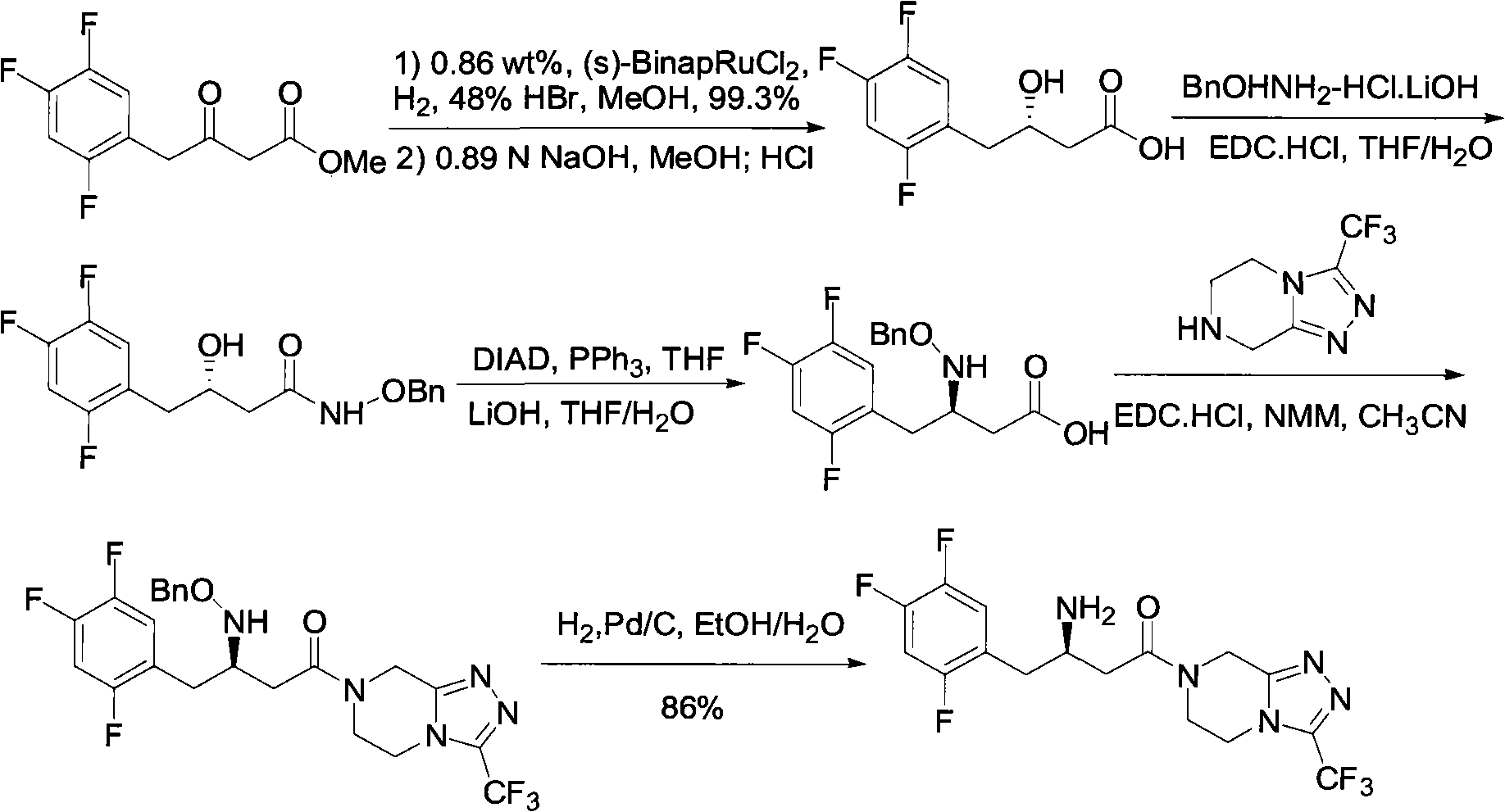
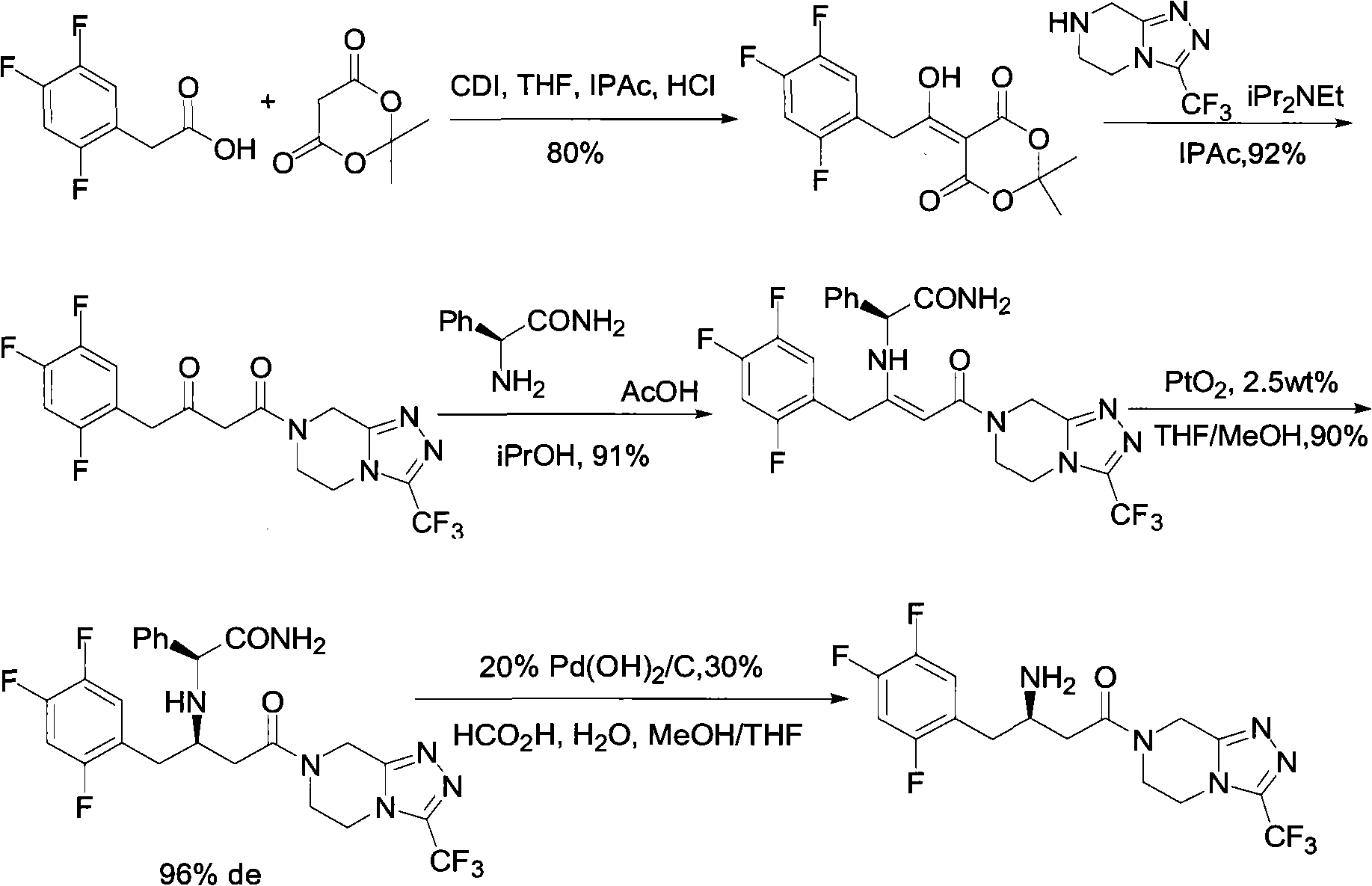
Sitagliptin intermediates as well as preparation method and application of intermediate
A synthesis method and reaction technology, applied in the intermediates of sitagliptin and its preparation and application fields, can solve problems such as expensive, difficult to control product quality, obvious amplification effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082]
[0083] Under nitrogen protection conditions, magnesium powder (2.4g, 99mmol) was added to a 250mL three-neck flask equipped with a thermometer, a constant pressure dropping funnel and a reflux condenser, 10mL THF was added, a grain of iodine was added, and 0.5g bromoethane was added to initiate the reaction. Add n-butyl chloride (8.4g, 90mmol) dissolved in 60mL THF to maintain reflux. After dripping, the oil bath is refluxed and kept for 4 hours, and the system is cooled to -10°C in an ice-salt bath. Fluorobromobenzene (21.8g, 103.5mmol), the system exothermic, the temperature was controlled below -5°C, after the dropwise addition was completed, the reaction was continued for 10min, and 1.6g of cuprous iodide was added. Then chlorinated propylene oxide III (9.3 g, 0.1 mol) dissolved in 10 mL of tetrahydrofuran was added dropwise to the system, and the reaction was exothermic. After reacting at 0° C. for 5 hours, 70 mL of saturated ammonium chloride aqueous solution ...
Embodiment 2
[0085]
[0086] The product obtained in the previous step was dissolved in 90mL THF, and 4.8g of 50% NaH was slowly added to this system, and the system was heated to 60°C for 5 hours of reaction. Thin-plate chromatography showed that the raw material had reacted completely (thin-plate chromatography conditions: petroleum ether: acetic acid Ethyl ester=10:1), standing to separate layers, extracting the aqueous phase with dichloromethane (30mL×1), combining the organic phases, drying over anhydrous magnesium sulfate, filtering, and removing the solvent from the filtrate to obtain 17g of light yellow oily liquid, two The yield of the first step reaction is 90%.
[0087] 1 H NMR (400MHz, CDCl 3 )δ: 7.12 (dd, J 1 =6Hz,J 2 =9Hz, 1H), 6.93(dd, J 1 =6Hz,J 2 =9Hz, 1H), 3.15(s, 1H), 2.81-2.83(m, 1H), 2.75-2.77(m, 2H).
[0088] ESI-MS: 189 (M + +1).
Embodiment 3
[0090]
[0091] The product obtained in Example 1 was dissolved in 90 mL of THF, and 5.4 g of NaOH in 20 mL of aqueous solution was added to this system. The system was heated to 60°C for 5 hours. Thin-plate chromatography showed that the raw materials were completely reacted (thin-plate chromatography conditions: petroleum ether: ethyl acetate = 10:1), and the layers were allowed to stand, and dichloromethane (30mL×1) was used for the aqueous phase. Extract, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and remove the solvent from the filtrate to obtain 16 g of light yellow oily liquid, and the yield of the two-step reaction is about 85%.
[0092] 1 H NMR (400MHz, CDCl 3 )δ: 7.12 (dd, J 1 =6Hz,J 2 =9Hz, 1H), 6.93(dd, J 1 =6Hz,J 2 =9Hz, 1H), 3.15(s, 1H), 2.81-2.83(m, 1H), 2.75-2.77(m, 2H).
[0093] ESI-MS: 189 (M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com