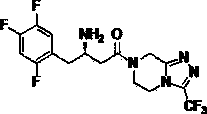
Preparation method of sitagliptin
A technology of sitagliptin and trifluoromethyl, applied in the field of pharmaceutical preparation, can solve the problems of unstable intermediate products, complicated operation, long time and the like, and achieve the effects of convenient industrial production, simple post-processing, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Compound methyl 3-oxo-3-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazine- Preparation of 7(8H)-yl)propyl Ester
[0043] Add 3-trifluoromethyl-[1,2,4]triazolo[4,3-a]piperazine hydrochloride (100.00g, 0.44mol) and methanol (300ml) into a 500mL three-necked reaction flask, The temperature of the system was lowered to -10°C, and a methanol solution of malonyl chloride (65.67 g, 0.48 moL) was slowly added dropwise at this temperature, stirred at room temperature, and the reaction of the raw materials was monitored by TLC. The solvent was recovered under reduced pressure, water (200ml) was added to the residue, the aqueous phase was extracted with ethyl acetate (200ml×2), the organic phases were combined, washed with water, and dried overnight with anhydrous magnesium sulfate. Filtration, the filtrate was concentrated to dryness under reduced pressure to obtain methyl 3-oxo-3-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a ]pyrazin-7(8H)-yl)...
Embodiment 2
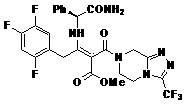
[0045] Example 2 Compound Methyl 3-oxo-2-(3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3-a] Preparation of pyrazine-7-carbonyl)-4-(2,4,5-trifluorophenyl)propyl ester
[0046] Add 2,4,5-trifluorophenylacetic acid (50.0g, 0.26mol) and acetonitrile (300ml), tert-butyryl chloride (10g), diisopropylethylamine (33.5g, 0.26mol) into a 500mL three-necked reaction flask and 4-dimethylaminopyridine (10g), after stirring for 1 hour, slowly drop into methyl 3-oxo-3-(3-(trifluoromethyl)-5,6-dihydro-[1, 2,4]Triazolo[4,3-a]pyrazin-7(8H)-yl)propyl ester (92g, 0.32moL) in acetonitrile solution, after dripping, slowly rise to 75°C to react, TLC monitoring until the raw material The response is over. Acetonitrile was recovered under reduced pressure, water (200ml) was added to the residue, the aqueous phase was extracted with ethyl acetate (200ml×3), the organic phases were combined, washed with water, and dried overnight with anhydrous magnesium sulfate. Filtration, and the filt...
Embodiment 3
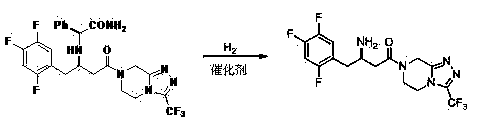
[0048] Example 3 Compound (S,E)-methyl 3-(2-amino-2-oxo-1-phenethylamino)-2-(3-(trifluoromethyl)-5,6,7,8 -Tetrahydro-1,2,4-triazolo[4,3-a]pyrazine-7-carbonyl l)-4-(2,4,5-trifluorophenyl)but-2-enol ester preparation of
[0049] Add methyl 3-oxo-2-(3-(trifluoromethyl)-5,6,7,8-tetrahydro-1,2,4-triazolo[4,3 -a] pyrazine-7-carbonyl)-4-(2,4,5-trifluorophenyl) propyl ester (51.0g, 0.11mol), isopropanol (150ml) and acetic acid (200ml), stirred at room temperature for 20 minute. (S)-Phenylglycineamide (21.0 g, 0.14 moL) was slowly added, and after the addition was completed, the reaction was slowly raised to reflux, and the reaction of the raw materials was monitored by TLC. Water (200ml) was added to the system, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, washed with water, and dried overnight. Filtration, and the filtrate was concentrated to dryness under reduced pressure to obtain (S, E)-methyl 3-(2-amino-2-oxo-1-phenethylamino)-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com