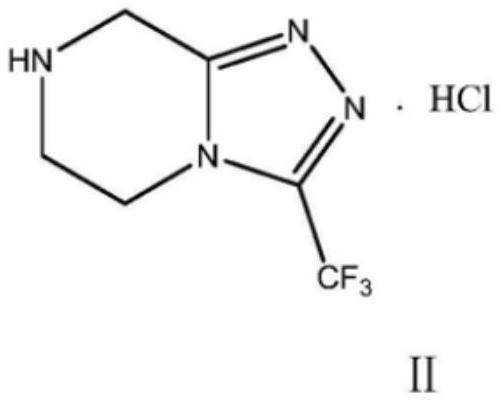
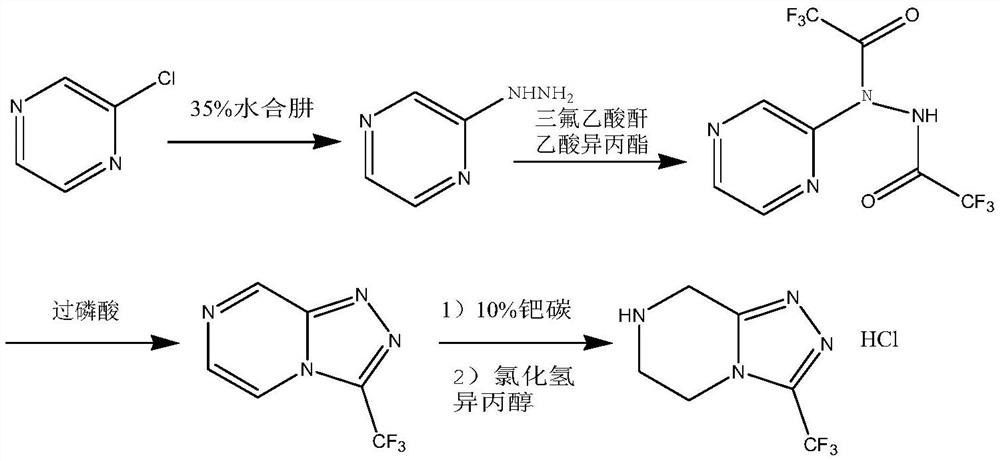
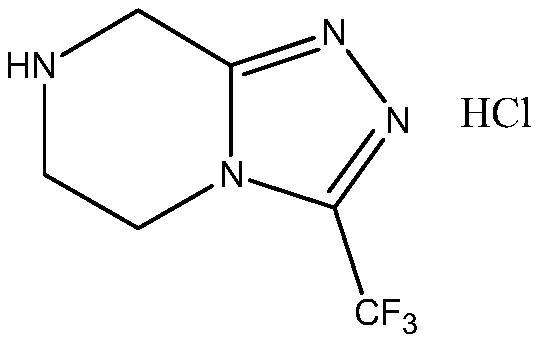
Preparation method of sitagliptin intermediate
A technology for sitagliptin and intermediates, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of poor product yield, poor environmental protection, poor reaction selectivity and the like, and achieves the effects of easy operation, easy handling and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Take 30.05g (0.50mol) of 1,2-ethylenediamine and dissolve it in 150ml of ethanol, stir evenly, cool down to 0-5°C, control the temperature and slowly add 61.28g (0.50mol) of ethyl chloroacetate dropwise, and control the temperature at 0 ~5°C, after dropping, stir for 5 hours, then slowly add 20% sodium ethoxide ethanol solution (containing 34.03g sodium ethoxide, 0.50mol) dropwise, temperature control ≤ 25°C, stir at room temperature until complete reaction, After the reaction is finished, filter, collect the filtrate and carry out vacuum distillation to remove the solvent, add acetone to the concentrate and carry out recrystallization treatment, obtain the corresponding product wet product, dry to obtain 2-piperazinone 42.55g (0.425mol ), the yield is 85.0%, and the purity is more than or equal to 98.0%.
Embodiment 2
[0031] Take 50.06 g (0.50 mol) of 2-piperazinone and dissolve it in 500 ml of ethanol, add 31.29 g of hydrazine hydrate with a content of 80% (equivalent to 0.50 mol of hydrazine hydrate), heat up to reflux reaction and keep it overnight, and then add no 10 g of magnesium sulfate in water was dried and stirred for 30 minutes, suction filtered, and the filtrate was collected for distillation and precipitation under reduced pressure to obtain 54.22 g (equivalent to 0.475 mol) of the oily substance of the intermediate product piperazine hydrazone, with a yield of 95.0% and a purity of ≥97.0% .
[0032] Synthesis of (N-[(2Z)-piperazin-2-ylidene]trifluoroacetylhydrazine
[0033] Take 54.22g (0.475mol) of the piperazine hydrazone oil obtained above and dissolve it in 480ml of acetonitrile, cool down to 0-5°C, then slowly add 67.49g (0.475mol) of ethyl trifluoroacetate dropwise, after the dropwise addition, slowly raise the temperature to 25°C, heat preservation reaction overnight, ...
Embodiment 3
[0036] Get 50.06 g (0.50 mol) of 2-piperazinone and dissolve it in 400 ml of ethanol, add 45.9 g of hydrazine hydrate with a content of 80% (equivalent to 0.75 mol of hydrazine hydrate), heat up to reflux reaction and keep it overnight, and then add no 15 g of magnesium sulfate in water was dried and stirred for 45 minutes, suction filtered, and the filtrate was collected for vacuum distillation and precipitation to obtain 54.56 g of the oily substance of the intermediate product piperazine hydrazone, with a yield of 95.3% and a purity of ≥97.8%.
[0037] Synthesis of (N-[(2Z)-piperazin-2-ylidene]trifluoroacetylhydrazine
[0038] Take 54.56g (0.478mol) of the piperazine hydrazone oil obtained above and dissolve it in 500ml of acetonitrile, cool down to 0-5°C, then slowly add 88.29g (0.621mol) of ethyl trifluoroacetate dropwise, after the dropwise addition, slowly raise the temperature to 23°C, heat preservation reaction overnight, then add 15g of sodium sulfate to dry, filter wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com