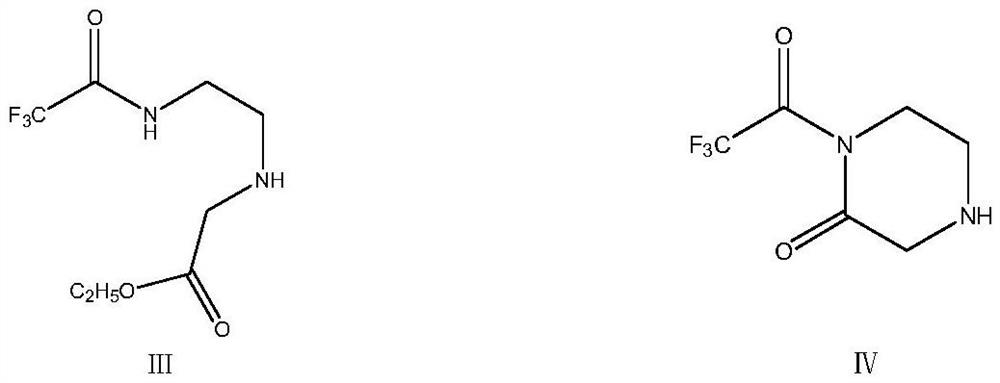Green synthesis method of sitagliptin intermediate
A sitagliptin and green synthesis technology, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problems of easy decomposition, inconvenient storage materials, poor environmental protection and the like, and achieves the effects of improving product yield, high product yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
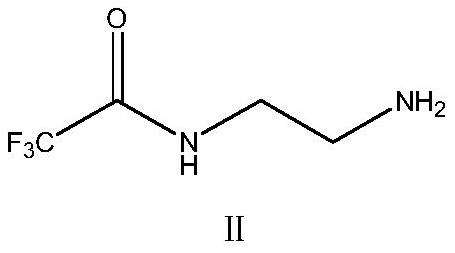
[0038] Synthesis of 2-Trifluoroacetamidoethylamine
[0039]Add 200ml of methyl tert-butyl ether into a 500ml clean three-necked bottle, then weigh 30.05g (0.50mol) of ethylenediamine and drop it into the three-necked bottle. During the dropping process, keep the temperature ≤ 25°C and stir after dropping Evenly, cool down to -5~0℃. Slowly add 71.04 g (0.50 mol) of ethyl trifluoroacetate, temperature control -5 ~ 0 ° C, after dropping, keep warm and stir for 4 ~ 6 hours, control ≥ 97% in sampling, after the reaction, add saturated saline for washing Twice, each time with 50ml saturated saline, the aqueous layer was extracted once with 50ml methyl tert-butyl ether, the collected organic phases were combined, added 15g of anhydrous sodium sulfate to dry for 1h, then, suction filtered, and the collected filtrate was reduced Pressure distillation removes solvent, obtains concentrate 2-trifluoroacetamidoethylamine, then adds 50ml ethanol and 200ml toluene to concentrate and dissolv...
Embodiment 2
[0041] The feed liquid containing the concentrate 2-trifluoroacetamidoethylamine obtained in Example 1 was transferred to another 500ml clean three-necked flask, and 61.28g (0.50mol) of ethyl chloroacetate was added dropwise at room temperature. Stir at room temperature for 1h, then slowly add 50.5g (0.50mol) of N-methylmorphine dropwise, raise the temperature to 50-55°C after dropping, control the temperature and carry out the reaction with heat preservation and stirring for 2h, after the end, directly continue to heat up to 90°C- Stir at 100°C for 5 hours to remove ethanol and generate the intermediate product 1-trifluoroacetyl-2-piperazinone. At the same time, the distilled ethanol can be collected. After the reaction is completed, add 100ml of water, stir for 30 minutes, stand still, and separate the liquid. The organic layer was collected, the aqueous layer was extracted once with 50ml of toluene, and the organic phases were combined to obtain a feed solution containing th...
Embodiment 3
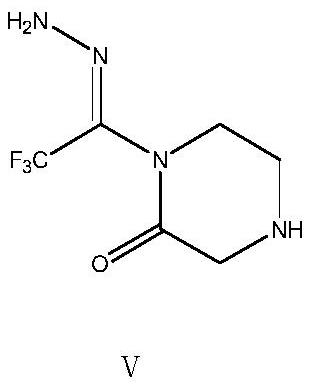
[0043] Transfer the feed solution obtained in Example 2 of the previous step to a 500ml clean three-necked flask, add 31.29g (0.50mol) of 80% hydrazine hydrate dropwise at room temperature, slowly raise the temperature to 50°C to 55°C after dropping, and keep stirring for 3h. After the reaction, cool down to room temperature, let stand, separate the liquid, collect the organic phase, transfer the organic phase into a 500ml three-necked flask, add 50g of 5A molecular sieve, and then raise the temperature to 50°C to 55°C, stir and fully react for 3 hours to form 1 -Trifluoroacetylhydrazino-2-piperazinone, after the reaction is completed, suction filtration, the collected organic phase is subjected to vacuum distillation and desolventization, and 200ml of methyl tert-butyl ether is added to perform beating treatment at room temperature for 2h, and then , slowly lower the temperature to 0-5°C for full crystallization, filter with suction to obtain the wet intermediate product 1-tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com