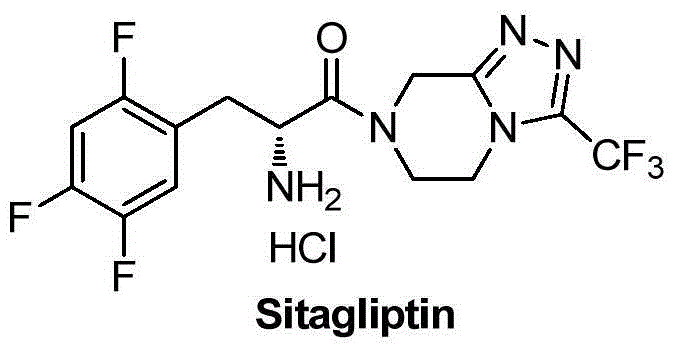
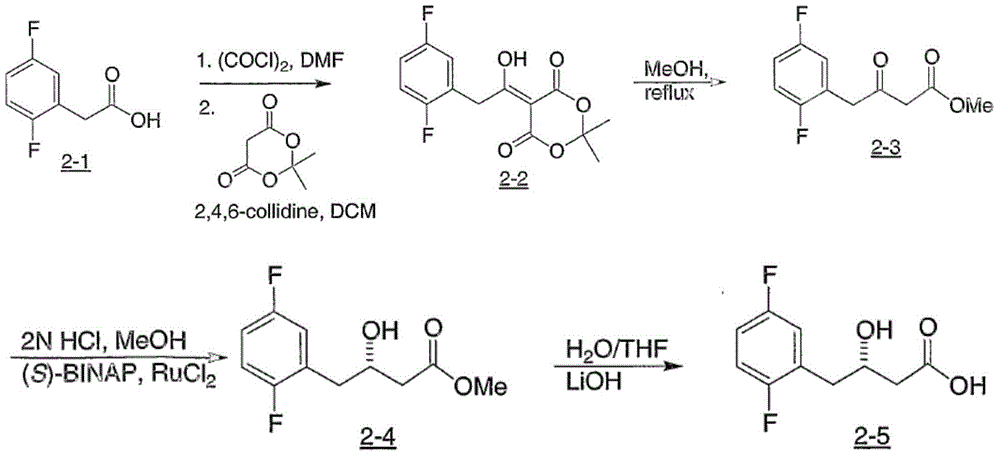
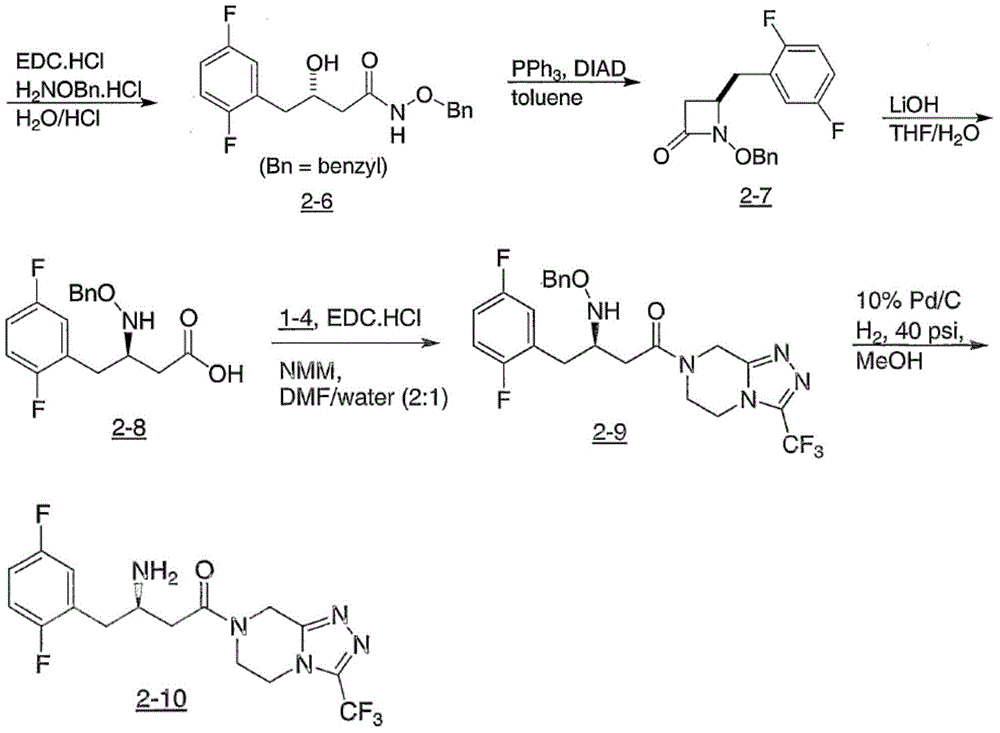
Intermediate for preparing Sitagliptin and preparation method and application of intermediate
A technology for sitagliptin and its use, which is applied to the preparation of an intermediate for sitagliptin and the field of preparation thereof, can solve the problems of complex reaction, harsh operating conditions, expensive starting materials and the like, and achieves high purity and high application value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the preparation of compound b
[0074]
[0075] Add the compound of formula 7 (1.3g, 0.01mol) and compound 9 (1.9g, 0.01mol) into a 50ml two-necked flask, add 20ml of dichloromethane to stir the solvent, add HOBt (1.49, 0.011mol) and EDC.HCl (2.9g, 0.015mol) the reaction mixture was stirred at room temperature for 8 hours, after the reaction was completed, 15mL NaHCO was added3 The aqueous solution and the organic phase were washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and purified by column chromatography to obtain 2.67 g of the compound of formula b with a yield of 89.1%.
[0076] LC-MS m / z=303(M-H) + .
Embodiment 2
[0077] Embodiment 2: the preparation of compound c
[0078]
[0079] Catalyst preparation:
[0080] N 2 Under protection, put 4g Dppp (1mmol, 0.1eq), 0.11g (0.5mmol, 0.05eq) palladium acetate, 4ml acetonitrile into a 20ml one-mouth bottle, replace with nitrogen for 3 times, stir, heat in a water bath to 58-62°C, keep warm 2.5 to 3.5 hours. After the heat preservation is completed, cool to 30-35°C to obtain the catalyst.
[0081] N 2 Under protection, put 3.0g (0.01mol, 1.0eq) of compound b and 10ml of acetonitrile into a 50ml two-neck flask, stir and mix evenly; add DBU: 3.8g (0.025mol, 2.5eq), dissolve clear; add 2,4, 5-trifluorobromobenzene: 2.32g (0.011mol, 1.1eq), stirring; N 2 Replaced 3 times, adding the catalyst prepared above, N 2 Replacement 3 times, N 2 Under protection, keep warm at 80°C for 20-26 hours. After the heat preservation is completed, TLC detects that the reaction is complete until the raw material point disappears. The reaction solution was wa...
Embodiment 3
[0083] Embodiment 3: the preparation of compound e
[0084]
[0085] Put 4.4g (0.01mol, 1.0eq) of compound d and 20ml of dichloromethane into the four-neck flask, cool down to 0-5°C, slowly add 1.8g (0.015mol, 1.5eq) of thionyl chloride dropwise, and control the temperature 0~5℃, after dropping, raise the temperature to 35~40℃ and keep it for 4~6h, the reaction is over. A dichloromethane solution of compound e was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com