A-D-A conjugated molecule of seven-fused-ring unit based on dibenzofuran and preparation method and application thereof
A benzodifuran, A-D-A technology, applied in the molecular field, can solve the problems of weak absorption, high cost, and difficult energy level regulation, and achieve the effects of structural unit modification, rich sources, and easy structural units.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
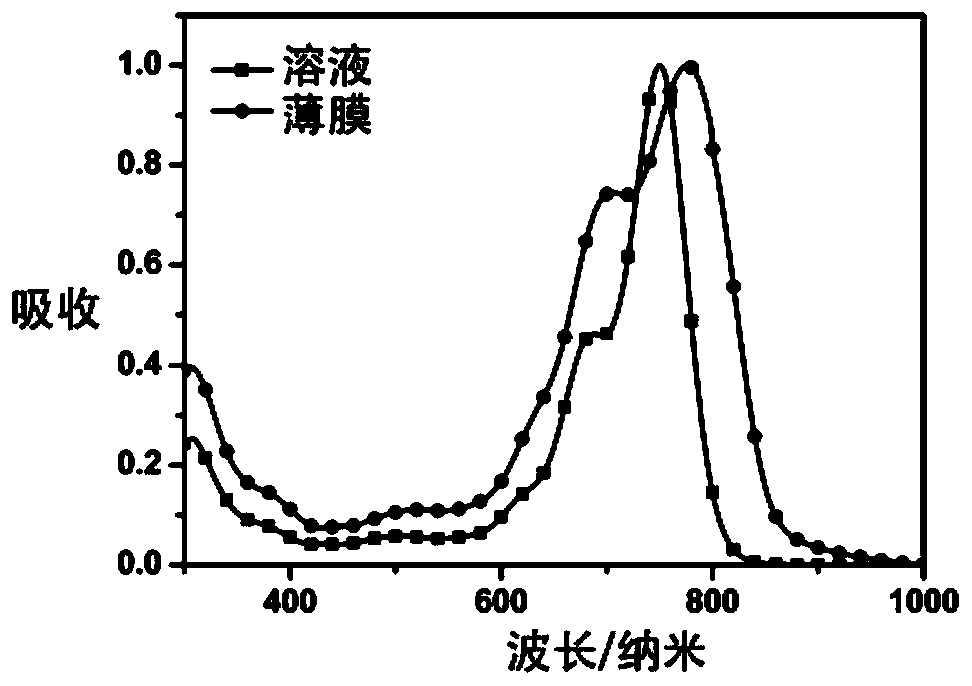
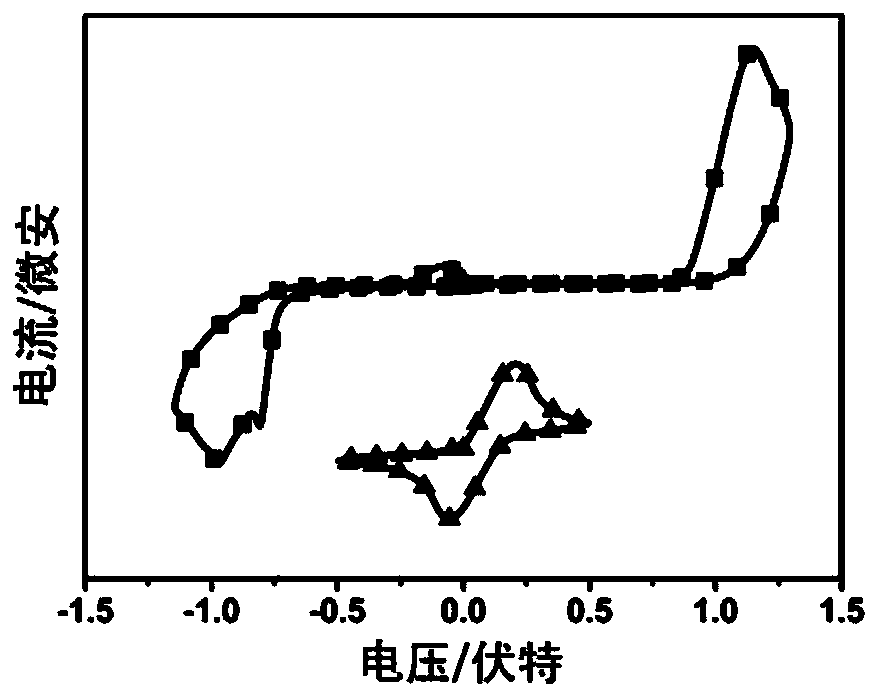
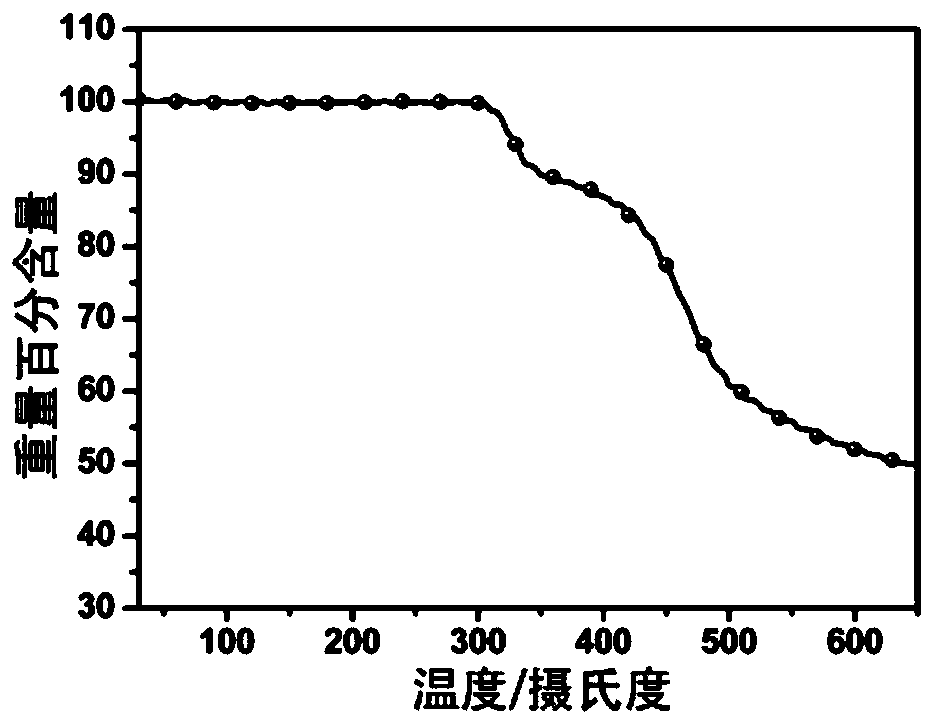
Image
Examples
Embodiment 1
[0045] see Figure 6 , Figure 6 It is the IFIC-4F synthetic route of the present invention. Such as Figure 6 As shown, this example shows the synthesis route of the A-D-A conjugated molecule based on the hepta-fused ring unit of benzodifuran in the following steps:
[0046] in:
[0047] R 1 represents 4-hexylphenyl, R 2 Represents 2-ethylhexyl;
[0048] The detailed synthetic steps of its each step product are as follows:
[0049] Step 1) Synthesis of compound 3: (4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b']difuran-2, 6-diyl)bis(thiophene-3-carboxylate):
[0050] In a dry 500mL two-necked round-bottom flask, compound 1, namely (4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4 ,5-b']difuran-2,6-diyl)bis(trimethylstannane) (2.93g, 3.36mmol), compound 2, namely ethyl 2-bromothiophene-3-carboxylate (2.37g , 10.08mmol) and the catalyst tetrakis(triphenylphosphine) palladium [Pd(PPh 3 ) 4 ] (0.031g, 0.0269mmol) were dissolved together in dry and purif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com