Organic electronic material containing dibenzoheterocycle substituted phenanthrene and anthracene and application of organic electronic material
A technology of organic electronic materials and diphenyl, which is applied in the direction of luminescent materials, organic chemistry, organic chemical methods, etc., can solve the problems of undisclosed compounds and application effects, and no mention of dibenzothiophene and application effects, etc., to achieve Improve luminous efficiency, enhance charge transport performance, synthesize simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
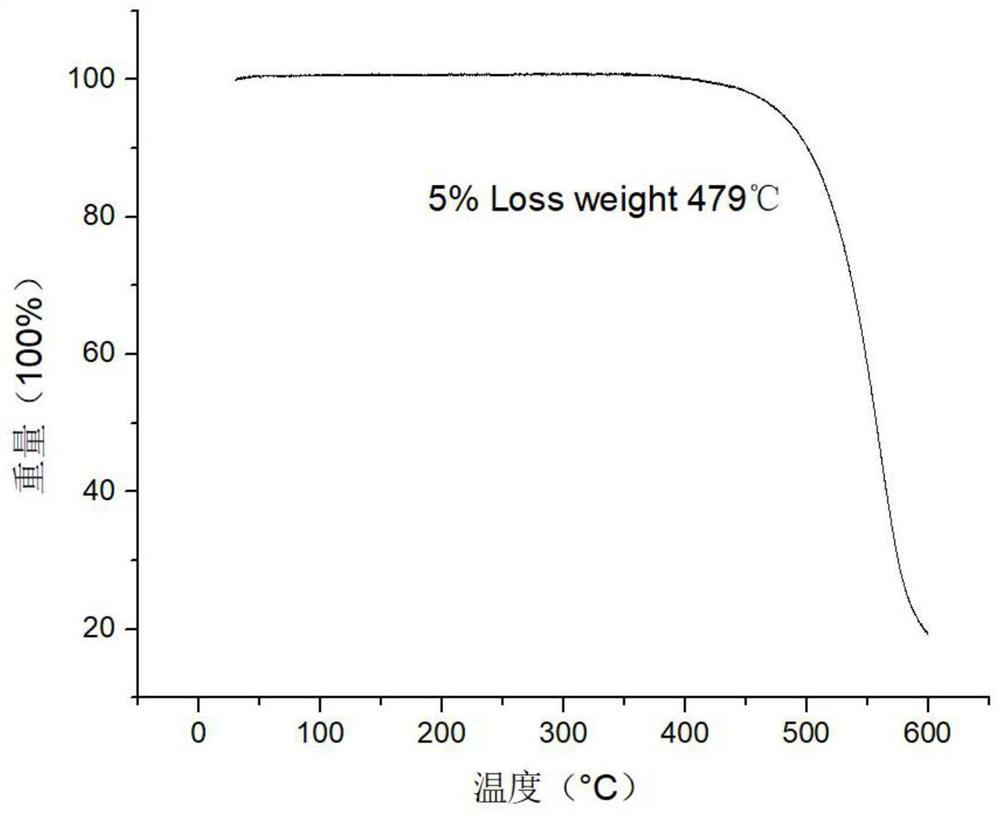
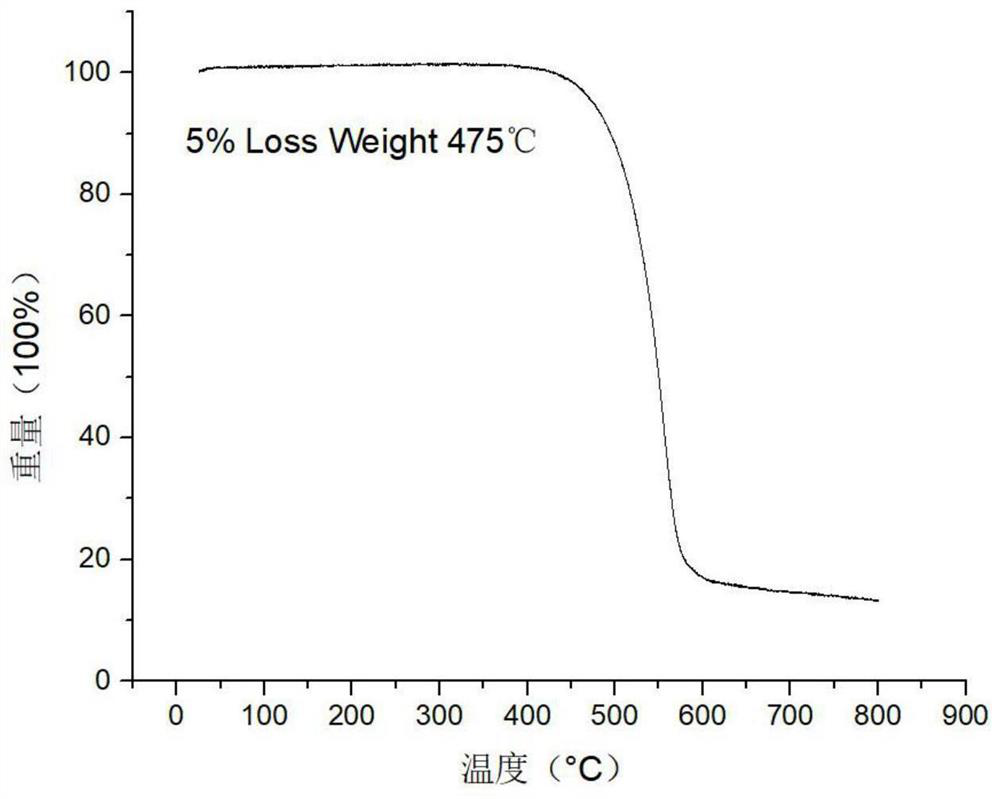
Image
Examples
Embodiment 1
[0053] The synthesis of embodiment 1 compound 2
[0054]
[0055] Synthesis of Intermediate 2-1
[0056] In a three-necked flask, add 9-bromo-2-chlorophenanthrene (10g, 34.4mmol), 1-dibenzofuran boronic acid (7.6g, 36mmol), potassium carbonate (9.3g, 68mmol), then add 100mL toluene, 50mL Ethanol and 50mL deionized water, add 0.5gPd(PPh 3 ) 2 Cl 2 , heated to reflux for 5 hours under the protection of nitrogen, cooled, a solid precipitated out, filtered, the filter cake was rinsed with ethanol, dried, the filter cake was recrystallized with toluene, filtered, and dried to obtain 9.6 g of a solid with a yield of 75%.
[0057] Synthesis of compound 2
[0058] In a three-necked flask, add intermediate 2-1 (9g, 23.8mmol), 9-(phenyl-d5)-10-anthraceneboronic acid (7.9g, 26.2mmol), potassium carbonate (6.9g, 50mmol), and then add 100mL of toluene, 50mL of ethanol and 50mL of deionized water, add 0.5g of palladium acetate, 1g of Xphos (2-dicyclohexylphosphine-2',4',6'-triisopro...
Embodiment 2
[0059] The synthesis of embodiment 2 compound 26
[0060]
[0061] Synthesis of Intermediate 26-1
[0062] In a three-necked flask, add 2-chloro-9-iodo-10-phenylphenanthrene (15g, 36.2mmol), 1-dibenzofuran boronic acid (8.1g, 38mmol), potassium carbonate (10.3g, 68mmol), and Add 150mL toluene, 70mL ethanol and 70mL deionized water, add 0.5g Pd(PPh 3 ) 2 Cl 2 , heated to reflux for 5 hours under the protection of nitrogen, cooled, solids precipitated, filtered, the filter cake was rinsed with ethanol, dried, the filter cake was recrystallized with toluene, filtered, and dried to obtain 13.6 g of solids, with a yield of 83%.
[0063] Synthesis of compound 26
[0064] In a three-necked flask, add intermediate 26-1 (6g, 13.2mmol), 9-(phenyl-d5)-10-anthraceneboronic acid (4.4g, 14.5mmol), potassium carbonate (3.4g, 15mmol), and then add 80mL toluene, 40mL ethanol and 40mL deionized water, add 0.3g palladium acetate, 0.6g Xphos, heat to reflux for 5 hours under the protectio...
Embodiment 3
[0066] The synthesis of embodiment 3 compound 27
[0067]
[0068] In a three-necked flask, add intermediate 26-1 (6g, 13.2mmol), 9-(2-naphthyl)-10-anthraceneboronic acid (5.1g, 14.5mmol), potassium carbonate (3.4g, 15mmol), and then add 80mL toluene, 40mL ethanol and 40mL deionized water, add 0.3g palladium acetate, 0.6g Xphos, heat to reflux for 5 hours under the protection of nitrogen, cool, there is solid precipitation, filter, rinse the filter cake with ethanol, dry, and use the filter cake Toluene was recrystallized, filtered, and dried to obtain 5.8 g of solid, with a yield of 61%.
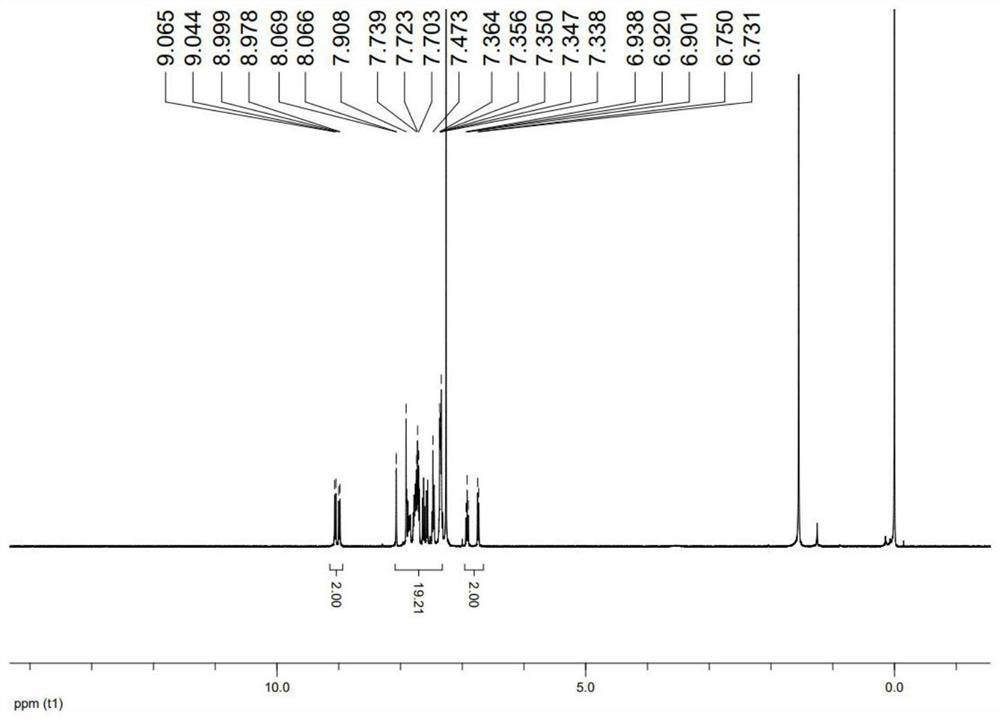
[0069] 1 H NMR (400MHz, CDCl 3 ,δ): 9.01-9.16 (m, 2H), 7.30-8.09 (m, 25H), 6.72-7.10 (m, 7H). HRMS(ESI,m / z):[M+H] + :723.2657.
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com