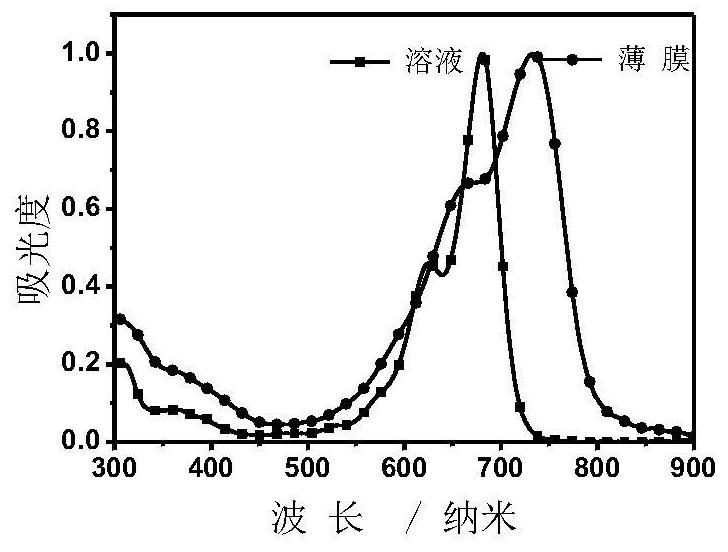
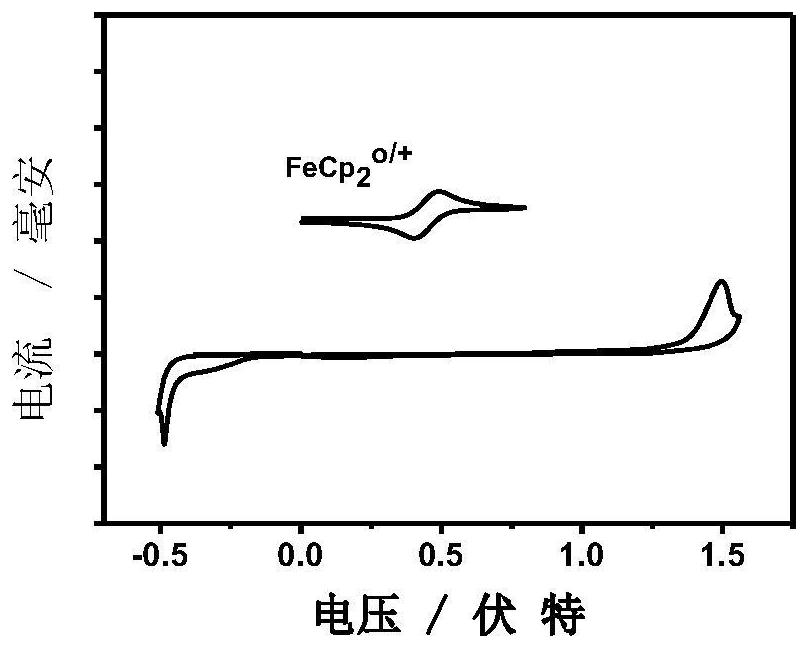
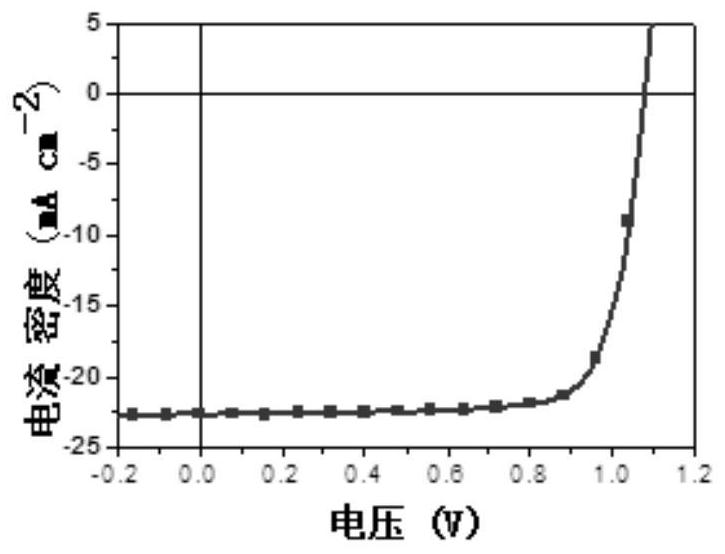
Application of multiple fused ring conjugated macromolecules in perovskite solar cells
A solar cell and perovskite technology, applied in circuits, electrical components, photovoltaic power generation, etc., can solve the problems of complex and tedious purification process, poor device stability, difficult energy level regulation, etc., to achieve strong light absorption, stability improvement, Hysteresis improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] The preparation method of the above-mentioned multi-fused ring conjugated macromolecules preferably includes: in the presence of a basic compound and in an organic solvent, dehydration condensation of the compound represented by the following formula (2) and the compound represented by the formula (a) Reaction, obtains the compound shown in formula (1); Wherein,
[0075]
[0076] Formula (a) is selected from one or more of the following compounds:
[0077]
[0078] In this method, the groups A-B, R 1 -R 14 As described above, the present invention will not be repeated here.
[0079] Wherein, the compound shown in formula (2) can be selected according to the structure of the poly-fused ring conjugated macromolecule above, preferably, the compound shown in formula (2) is one or more of the following formulas:
[0080]
[0081] Specific examples of compounds represented by formula (2) can be, for example, one or more of the following formulae:
[0082] Formula...
preparation example 1
[0150] This preparation example is used to illustrate the preparation method of the compound represented by formula (2-5-1).
[0151]
[0152] As shown in the above reaction formula, the compound represented by formula ID (131mg, 0.2mmol; purchased from Suzhou Nakai Technology Co., Ltd.), tetrahydrofuran (20mL) was added to the reaction vessel, argon was blown, and stirred at -78°C for 1h . Slowly add n-butyl lithium (0.38mL, 0.6mmol, 1.6M) dropwise, stir at -78°C for 2h, add N,N-dimethylformamide (36.6mg, 0.5mmol), and slowly return the reaction product to At room temperature (about 25°C), stir overnight (about 12h). Then add water (0.2mL) to quench, use saturated brine and dichloromethane to extract, use magnesium sulfate to dry, spin dry, the precipitate that obtains uses silica gel chromatographic column (using 200-300 purpose silica gel, eluent is volume ratio (2:1 petroleum ether / dichloromethane) for chromatographic separation to obtain a bright yellow solid (91 mg,...
preparation example 2
[0154] This preparation example is used to illustrate the preparation method of the compound represented by formula (2-9-2).
[0155]
[0156] As shown in the above reaction formula, the compound represented by the formula IBT (226mg, 0.2mmol; purchased from Suzhou Nakai Technology Co., Ltd.), tetrahydrofuran (35mL) was added to the reaction vessel, argon was blown, and stirred at -78°C for 1h . Slowly add n-butyllithium (0.4mL, 0.64mmol, 1.6M) dropwise, stir at -78°C for 1.5h, add N,N-dimethylformamide (65.8mg, 0.9mmol), and slowly return the reaction product to to room temperature (about 25°C), and stirred overnight (about 12h). Then add water (0.4mL) to quench, use saturated brine and dichloromethane to extract, use magnesium sulfate to dry, spin dry, the precipitate that obtains uses silica gel chromatographic column (using 200-300 purpose silica gel, eluent is volume ratio (1:1 petroleum ether / dichloromethane) for chromatographic separation to obtain an orange-red so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com