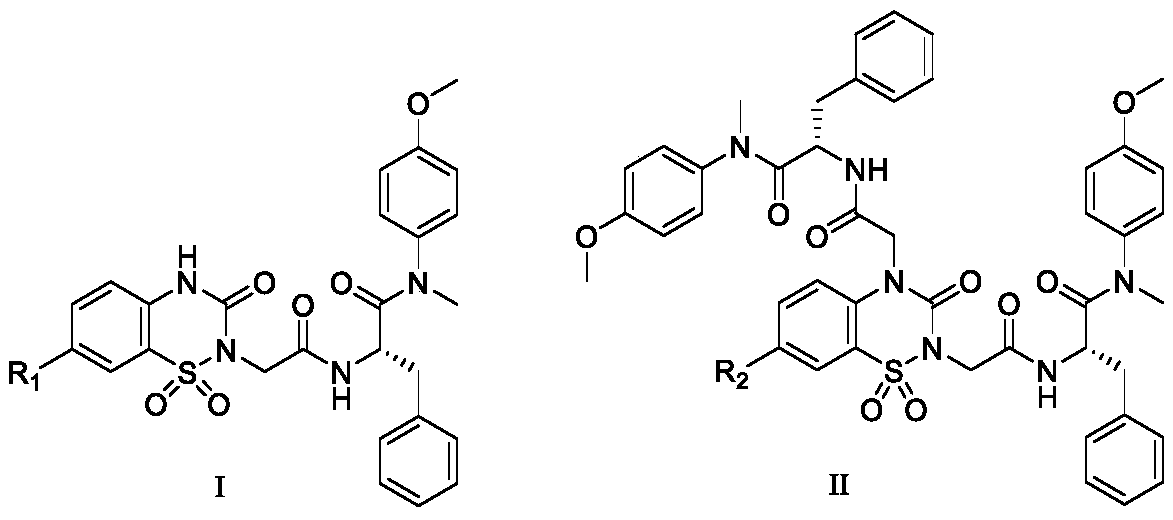
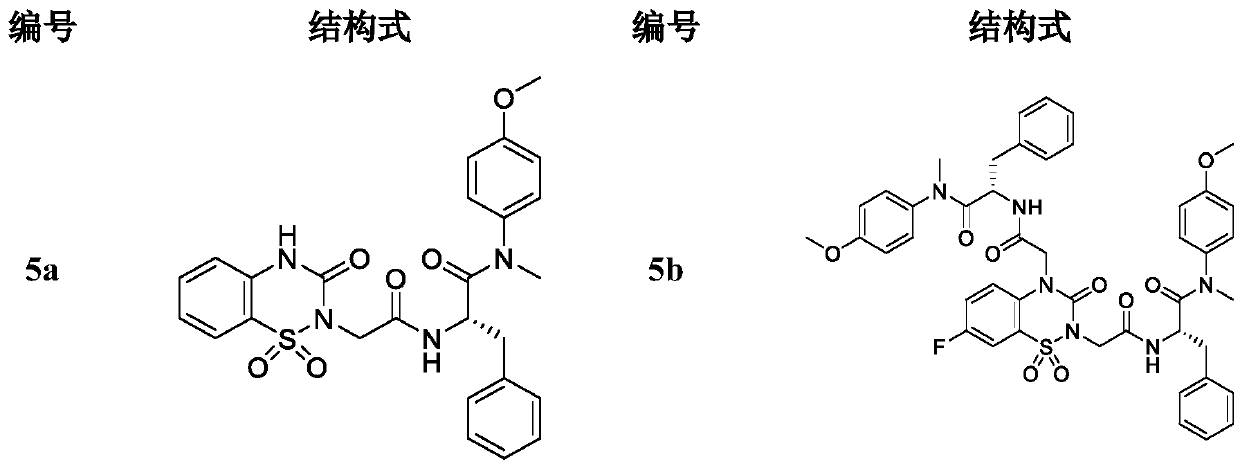
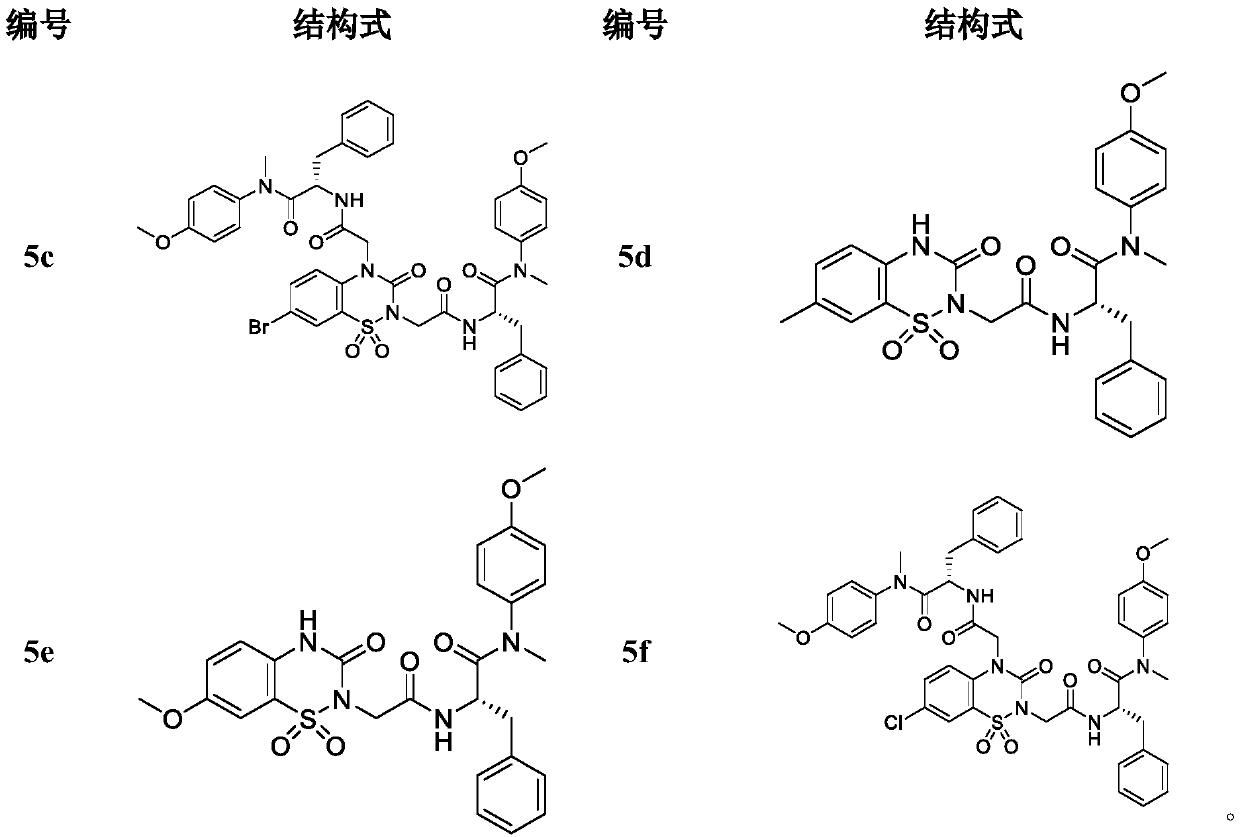
Phenylalanine derivative containing benzothiadiazine-3-ketone 1,1-dioxide and preparation method and application thereof
A technology of benzothiadiazine and dioxide, which is applied in the field of organic compound synthesis and pharmaceutical application, can solve the problems of low curative effect, poor drug-like properties, easy induction of drug resistance, etc., and achieve high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: (S)-(1-((4-methoxyphenyl)(methyl)amino)-1-oxo-3-phenylpropan-2-yl)carbamate tert-butyl ester (2 ) preparation
[0044] Starting materials Boc-L-phenylalanine (1) (2.90g, 10.93mmol, 1.5eq.), 1H-benzotriazol-1-yloxytripyrrolidinyl hexafluorophosphate (5.69g, 10.93mmol, 1.5eq.) was added to 20mL of dichloromethane, stirred for 30min under ice-bath conditions; then N,N-diisopropylethylamine (3.61mL, 21.87mmol, 3eq.) and N-methyl -4-Aminoanisole (1.0g 7.29mmol, 1eq.), remove the ice bath and transfer to room temperature to stir, TLC monitoring; after 6h, the reaction is complete, the solvent is evaporated under reduced pressure, and then saturated bicarbonate is added to the residue in the bottle Sodium solution 40mL, dichloromethane 40mL extraction, take the organic phase, add 1N HCl solution 40mL to wash, take the organic phase, add saturated sodium chloride solution 40mL to wash, the organic phase is dried with anhydrous sodium sulfate, filter, and the filt...
Embodiment 2
[0049] Example 2: Preparation of (S)-2-amino-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (3)
[0050] Intermediate 2 (4.0g, 10.40mmol, 1.0eq.) was added to 30mL of dichloromethane, then trifluoroacetic acid (3.86mL, 52.02mmol, 5.0eq.) was slowly added to this solution, stirred at room temperature, monitored by TLC After 1h, the reaction was completed, then the pH of the reaction solution was adjusted to 7 with saturated sodium bicarbonate solution, 40 mL of dichloromethane was added for extraction, the organic phase was separated, washed with saturated sodium chloride solution (20 mL × 3 times), and dried over anhydrous sodium sulfate , filtered, and concentrated under reduced pressure to obtain 2.36 g of crude product of intermediate (S)-2-amino-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (3), yellow oil material with a yield of 80%.
[0051] Spectral data:
[0052] 1 H NMR (400MHz, DMSO-d 6 )δ7.29–7.13(m,3H,Ph-H),7.03–6.75(m,6H,Ph-H),3.77(s,3H,OCH 3 ),3.44–3.3...
Embodiment 3
[0055] Example 3: Preparation of intermediate (S)-2-(2-bromoacetyl)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (4)
[0056] Bromoacetic acid (117mg, 0.84mmol, 1.2eq.), O-(7-azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate ( 401mg, 1.06mmol, 1.5eq.) was added to 15mL of dichloromethane, stirred in ice bath for 1h; then intermediate 3 (200mg, 0.70mmol, 1eq.) and N,N-diisopropyl Ethylamine (232μL, 1.41mmol, 2eq.), after removing the ice bath, stirred at room temperature, monitored by TLC; after 6h, the reaction was completed, the solvent was evaporated under reduced pressure, and the silica gel column chromatography (eluent EA:PE=1:4 + 2.5% triethylamine) to the intermediate (S)-2-(2-bromoacetyl)-N-(4-methoxyphenyl)-N-methyl-3-phenylpropanamide (4) 190 mg, white oil, yield 68%.
[0057] Spectral data: ESI-MS: m / z 405.4(M+1).C 19 h 21 BrN 2 o 3 [404.1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com