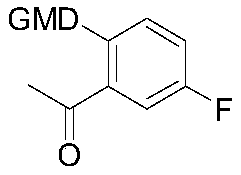
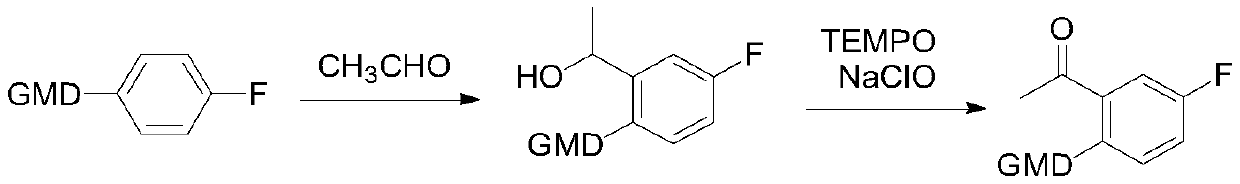
Preparation method of acetylation intermediate of (S)-5-fluoro-3-methylisobenzofuran-3-one
A technology of methyl isobenzene and intermediates, which is applied in the field of preparation of pharmaceutical intermediates, can solve problems such as low economic benefits, difficult industrial production, and long reaction steps, and achieve the goals of reducing environmental pollution, improving product quality, and enhancing acidity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) In a four-neck flask equipped with a thermometer and a stirrer, add 1.2 mol of potassium carbonate to dissolve in 3 times the volume of water, then add 5 times the weight of toluene and 1.2 mol of N,N-diisopropylamine reaction solution , start stirring, control the reaction temperature at 10-20°C, and start to add 1.0mol p-fluorobenzoyl chloride dropwise. After 2 hours of reaction, the reaction liquid is separated, and the organic phase is spin-dried to obtain intermediate a with a yield of 95% and a purity of 99%. %.
[0035] The structure of intermediate a is as follows:
[0036]
[0037] NMR data: 1 H NMR (400MHz, CDCl 3 ):δ7.29-7.34(m,2H),7.04-7.10(m,2H),3.67(s,2H),1.33(s,12H)ppm;
[0038] (2) Add 1.2 mol of n-butyllithium THF solution dropwise to 4 times the weight of intermediate a, and control the temperature at -70 to -60°C. After about 1 hour of reaction, add dropwise to 1.0 mol of N,N - In dimethylacetamide and 1 times weight THF solution, add dropw...
Embodiment 2
[0043] (1) In a four-neck flask equipped with a thermometer and a stirrer, add 1.2 mol of potassium carbonate to dissolve in 3 times the volume of water, then add 5 times the weight of toluene and 1.2 mol of N,N-diisopropylamine reaction solution , start stirring, control the reaction temperature at 10-20°C, start to drop 1.0mol of p-fluorobenzoyl chloride, react for 2 hours, separate the reaction solution, and spin dry to obtain intermediate a with a yield of 95% and a purity of 99%.
[0044] (2) Add 1.2 mol of n-butyllithium THF solution dropwise to 4 times the weight of intermediate a, and control the temperature at -70 to -60°C. After about 1 hour of reaction, add the reaction solution dropwise to 1.4 mol In N,N-dimethylacetamide and 1 times weight THF solution, add dropwise for 0.5h, keep it warm for 2h, add dilute hydrochloric acid dropwise to quench, spin the reaction solution to dryness, filter, and recrystallize with 3 times weight methanol, then Acetylated intermedia...
Embodiment 3
[0046] (1) In a four-neck flask equipped with a thermometer and a stirrer, add 1.2 mol of potassium carbonate to dissolve in 3 times the volume of water, then add 5 times the weight of toluene and 1.2 mol of N,N-diisopropylamine reaction solution , start stirring, control the reaction temperature at 10-20°C, start to drop 1.0mol of p-fluorobenzoyl chloride, react for 2 hours, separate the reaction solution, and spin dry to obtain intermediate a with a yield of 95% and a purity of 99%.
[0047] (2) Add 1.2 mol of n-butyllithium THF solution dropwise to the THF solution of 4 times the weight of intermediate a, and control the temperature at -70 to -60°C. After about 1 hour of reaction, add dropwise to 1.8 mol of N,N - In dimethylacetamide and 1 times weight THF solution, add dropwise for 0.5h, keep warm for 2h, add dropwise dilute hydrochloric acid to quench, spin the reaction solution to dryness, filter, and recrystallize with 3 times weight methanol to obtain acetyl Compound i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com