N-heterocyclic benzophenone derivative containing hydrogenated phenothiazine group and preparation method and application thereof
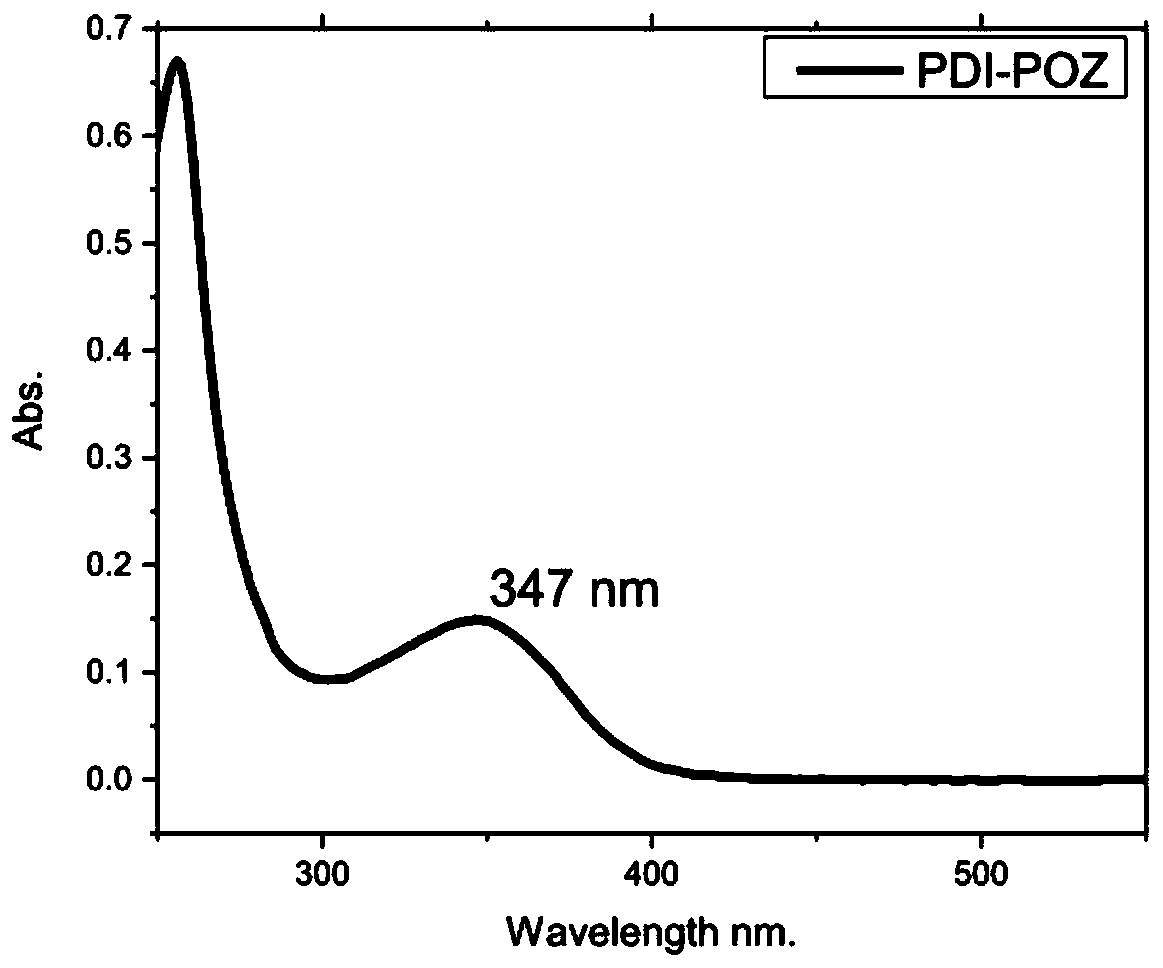
A technology based on phenothiazine and benzophenone, which is applied in the field of N-heterocyclic benzophenone derivatives and its preparation, can solve problems such as poor solubility, low luminous intensity, and insufficient thermal stability, and achieve good Application prospect, high fluorescence quantum yield, effect of unique AIE effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
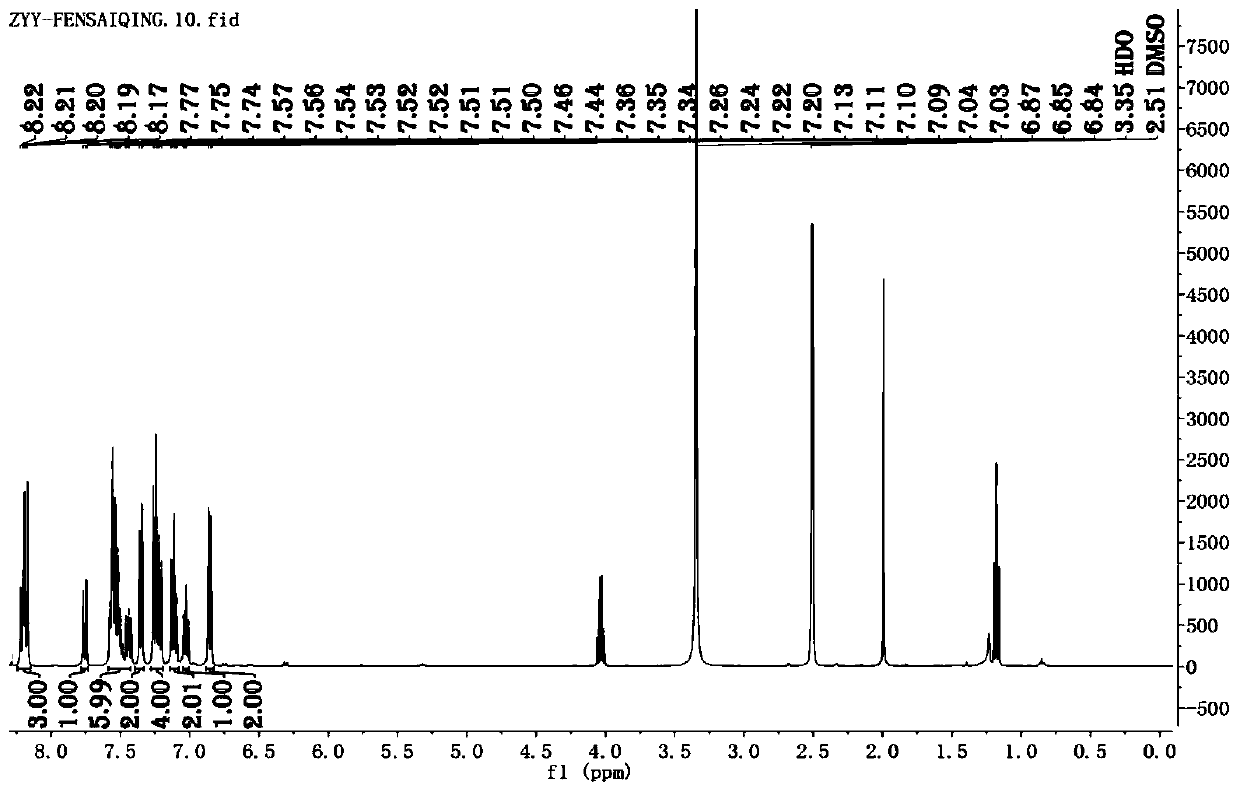
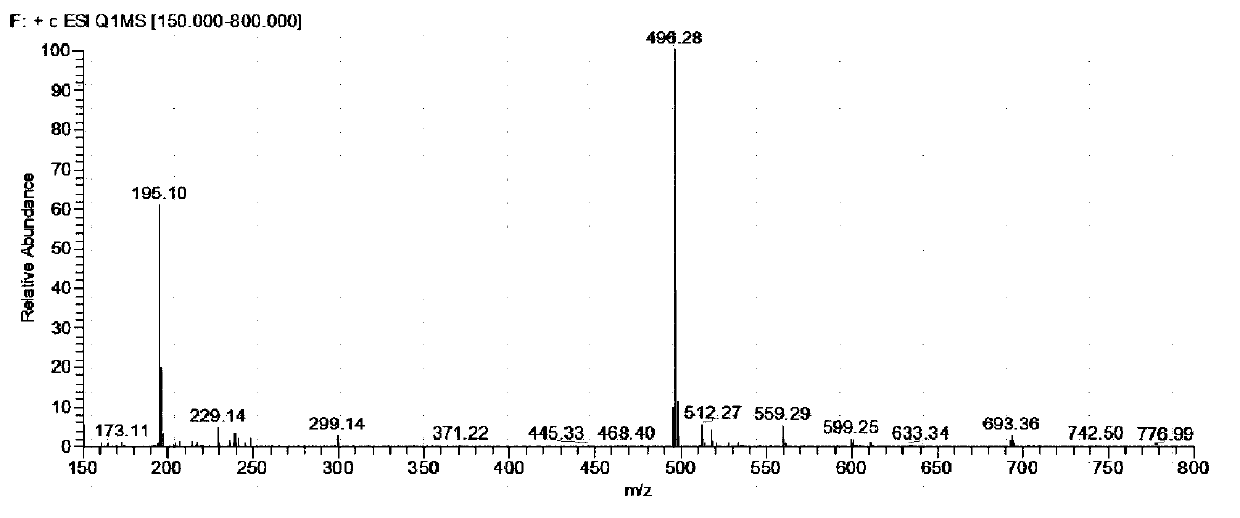
[0048] A N-heterocyclic benzophenone derivative containing a hydrophenothiazine group, i.e. a compound of formula (I), prepared by the following method:
[0049] (1) Preparation of (4-bromophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methanone:
[0050]
[0051]Weigh 43 mg of (E)-1-(4-bromophenyl)-3-phenylprop-2-en-1-one, 30 mg of 2-aminopyridine iodine 60 mg and 2 mL of dichloroethane in a 10 mL sealed tube, Carry out a Michael ring closure reaction at a temperature of 120°C to obtain (4-bromophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methanone after treatment;
[0052] (2) Preparation of formula (I) compound:
[0053]
[0054] Weigh (4-bromophenyl)(2-phenylimidazo[1,2-a]pyridin-3-yl)methanone 180mg, 10-hydro-phenothiazine 115mg, potassium tert-butoxide 80mg, tri-tert Put 4mg of butylphosphine, 5.5mg of palladium acetate and 5mL of toluene in a sealed tube, stir to remove the air in the device and fill it with nitrogen protection, heat, stir and reflux at 130°C for 15h ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com