Radical-polymerizable resin composition
A technology of resin composition and polymeric compound, applied in the direction of coating, primer, etc., can solve the problems of the influence of the curing mechanism itself, the performance reduction of the adhesive, and the damage to the appearance of the cured product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0411] Hereinafter, although this invention is demonstrated based on an Example, this invention is not limited to an Example.
[0412] The raw materials used for the manufacture of each radically polymerizable resin composition (hereinafter, also referred to as "resin composition") in Examples 1 to 11 and Comparative Examples 1 to 3 are as follows.
[0413]
[0415] Cobalt octanoate (manufactured by Toei Chemical Co., Ltd., cobalt hexanoate, content of cobalt in the total product 8% by mass, molecular weight 345.34)
[0417] Manganese octoate (manufactured by Toei Chemical Co., Ltd., manganese caproate, content of manganese in the total product 8% by mass, molecular weight 341.35)
[0418]
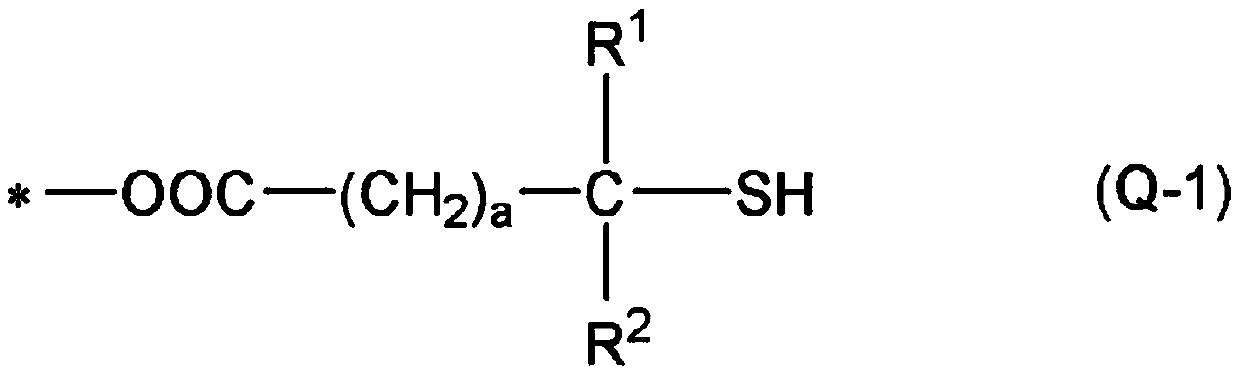
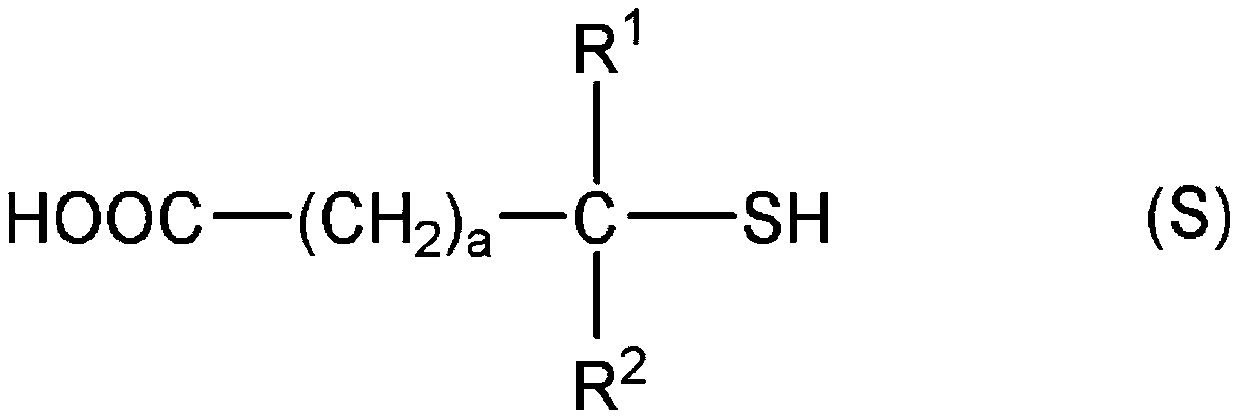
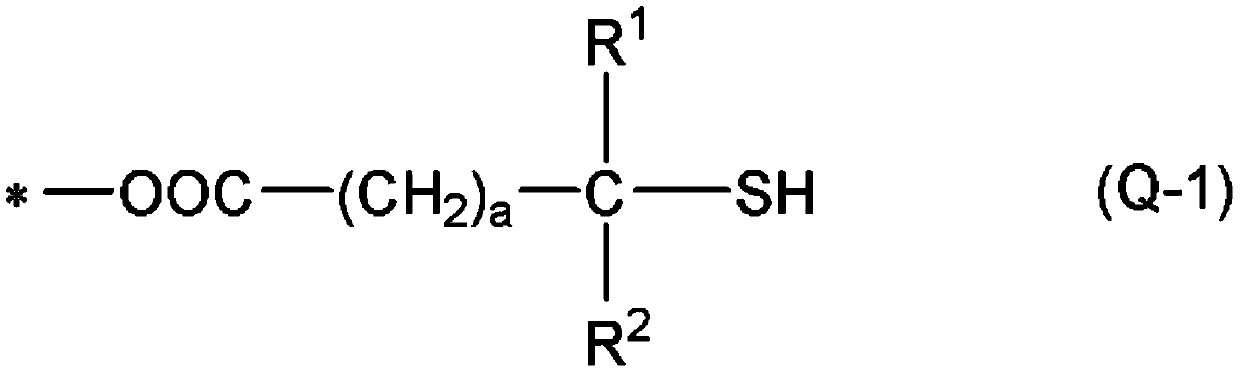
[0419] In addition, "monofunctional" in the following thiol compounds means that the number of mercapto groups in the molecule is one, and each numerical value of "bifunctional", "trifunctional", and "tetrafunctional" means that...
Synthetic example 1
[0453] Synthesis example 1: Synthesis of thiol-modified polycarbonate diol (B1-2)
[0454] 500 g (500 mmol) of C-1090 (manufactured by Kuraray Co., Ltd., molecular weight: 1,000, diol components: 3-methyl-1,5-pentanediol and 1,6-hexanediol) as a polycarbonate polyol, 3 -144 g (1198 mmol) of mercaptobutyric acid (manufactured by Showa Denko Co., Ltd.), 19 g (99.8 mmol) of p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.), and 500 g of toluene (manufactured by Junsei Chemical Co., Ltd.) were added to a 2L flask , install the Dean-Stark apparatus and cooling tubes.
[0455] After reducing the pressure in the reaction system to 73.3 kPa (550 mmHg), it heated using a 120 degreeC oil bath, stirring the content. After stirring for 7 hours, it was left to cool to room temperature, and the reaction liquid was neutralized with a 5% by mass aqueous sodium bicarbonate solution. The organic layer was further washed three times with ion-exchanged water...
Synthetic example 2
[0456] Synthesis example 2: Synthesis of mercaptan-modified product (B1-3) of dimer acid polyester polyol
[0457] 110 g (55 mmol) of Priplast1838-LQ-(GD) (manufactured by CRODA, molecular weight 2,000, hydroxyl groups at both ends) and 15.8 g (132 mmol) of 3-mercaptobutyric acid (manufactured by Showa Denko Co., Ltd.) ), 7.6 g (40 mmol) of p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.), and 150 g of toluene (manufactured by Junsei Chemical Co., Ltd.) were put into a 500 mL flask, and a Dean-Stark apparatus and a cooling tube were installed.
[0458] After reducing the pressure in the reaction system to 73.3 kPa (550 mmHg), it heated using a 120 degreeC oil bath, stirring the content. After stirring for 4 hours, it was left to cool to room temperature, and the reaction liquid was neutralized with a 9% by mass aqueous sodium bicarbonate solution. Furthermore, the organic layer was washed three times with ion-exchanged water, and the solv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com