Production method of dodecalactam
A technology of laurolactam and cyclododecanone is applied in the production field of high-purity laurolactam to achieve the effects of less by-products, not easy to agglomerate, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
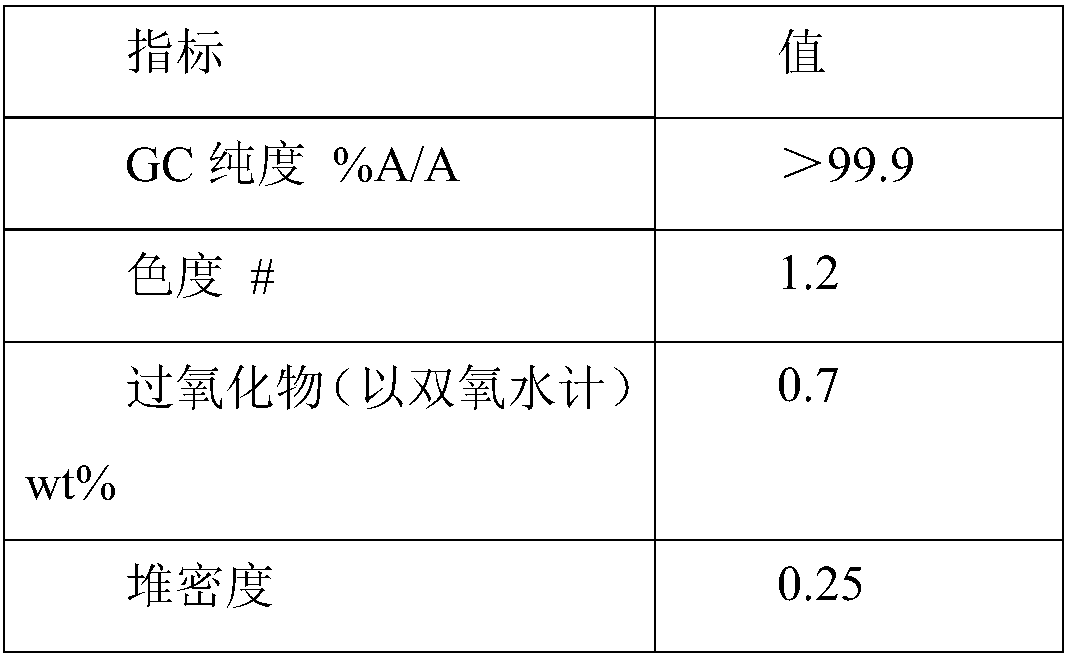
[0043] Step 1: 180 g of hydroxylamine sulfate (12.5 wt%) aqueous solution was added with ammonia to neutralize to pH 6-7 to obtain a hydroxylamine solution. In the round-bottomed bottle, 100 g of cyclododecanone was heated and melted, kept at 90° C., and the aforementioned hydroxylamine solution was added dropwise to the round-bottomed bottle with stirring. The dropwise addition time was about 10min. After the dropwise addition, the reaction was continued for 1h, and then 100 g of 1-cyclohexyl-n-tridecane was added to separate the solution. The oil phase obtained after liquid separation contained 0.2% water and 100 ppm of inorganic salts.
[0044] Step 2: The aforementioned oil phase is subjected to vacuum rectification, and the conditions for the distillation of cyclododecanone are the pressure of 1kPa, the temperature of 110°C, and the theoretical plate number of the rectification column is not less than 20. About 45 g of cyclododecanone was obtained with a purity of 98.7 w...
Embodiment 2
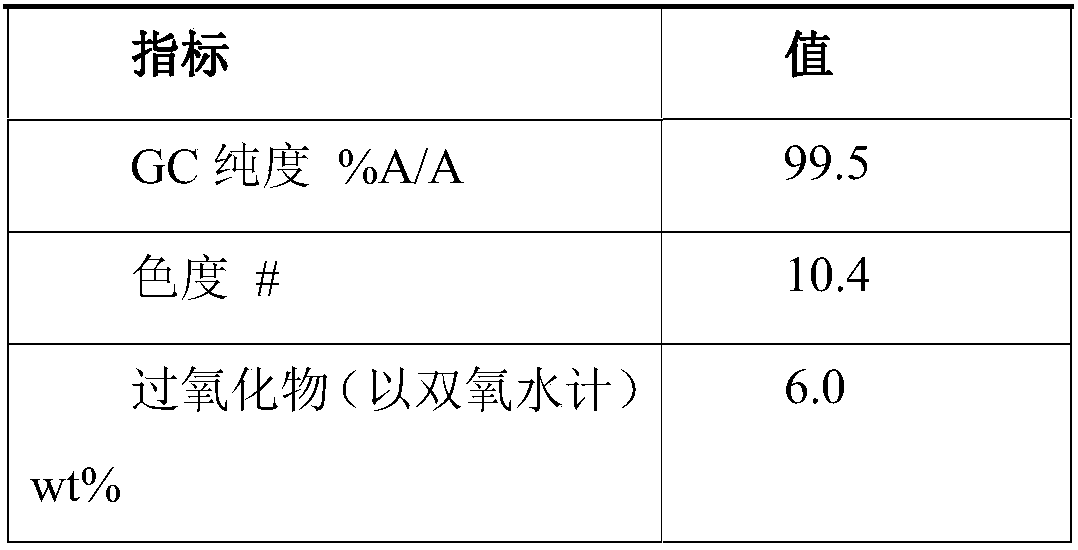
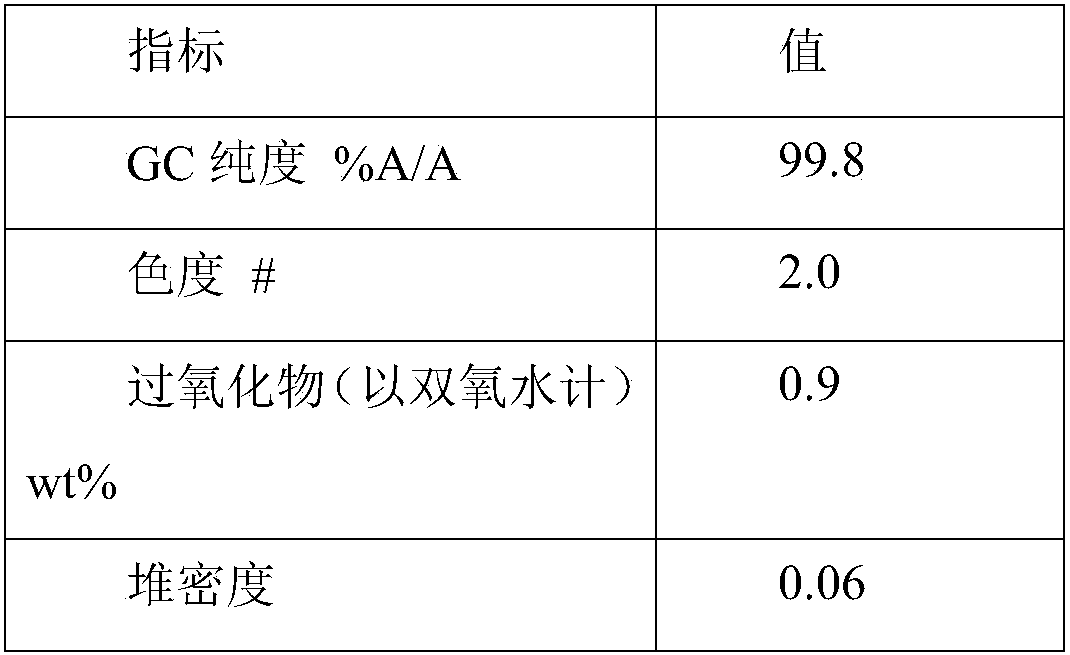
[0067] Step 1: 100 g of hydroxylamine sulfate (12.5 wt%) aqueous solution was added with ammonia to neutralize to pH 6-7 to obtain a hydroxylamine solution. In the round-bottomed bottle, 100 g of cyclododecanone was heated and melted, kept at 90° C., and the aforementioned hydroxylamine solution was added dropwise to the round-bottomed bottle with stirring. The dropwise addition time was about 10min. After the dropwise addition was completed, the reaction was continued for 1h, and then 120 g of 4-isopropyl-n-decylcyclohexane was added to separate the liquids. The oil phase obtained after liquid separation contained 0.15% water and 80 ppm of inorganic salts.
[0068] Step 2: The aforementioned oil phase is subjected to vacuum rectification, and the conditions for the distillation of cyclododecanone are the pressure of 1kPa, the temperature of 110°C, and the theoretical plate number of the rectification column is not less than 20. About 65 g of cyclododecanone was obtained, wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com