Quality control method for multi-wavelength fusion fingerprints in production process of Shenyi capsule bulk drug
A quality control method and fingerprint technology, applied in the field of quality control of raw materials of traditional Chinese medicine, can solve problems such as not being able to meet the safety and controllability requirements of Shenyi Capsules, and achieve perfect quality control methods and safe and reliable control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
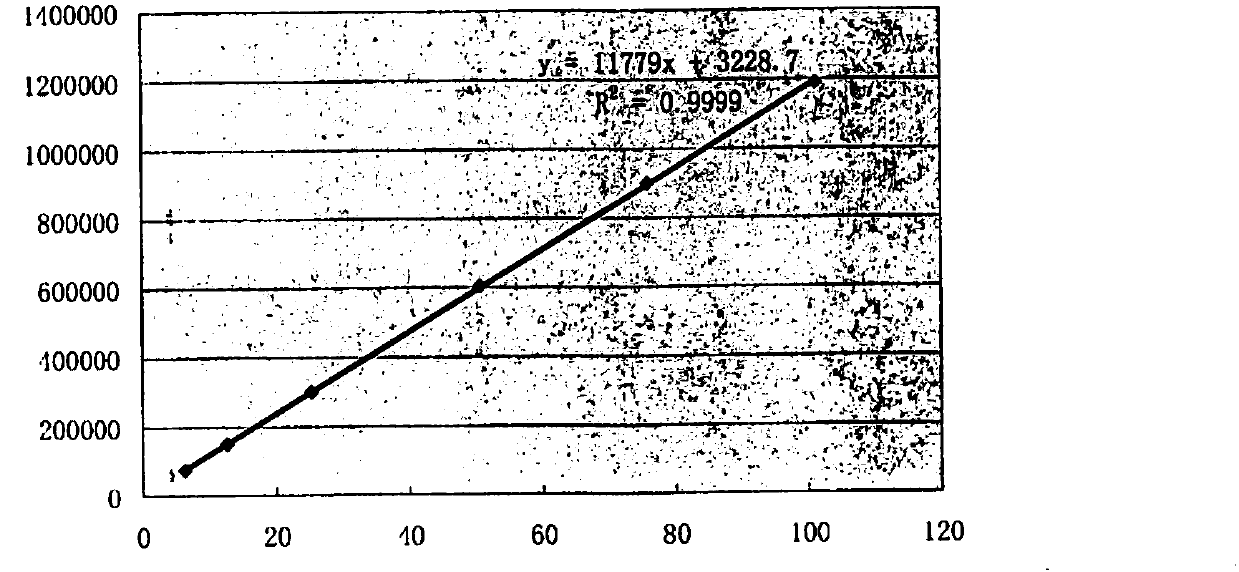
[0175] Example 2 Determination of the content of 20(R)-ginsenoside Rg3 of the Shenyi Capsule bulk drug whose batch number is 20160502
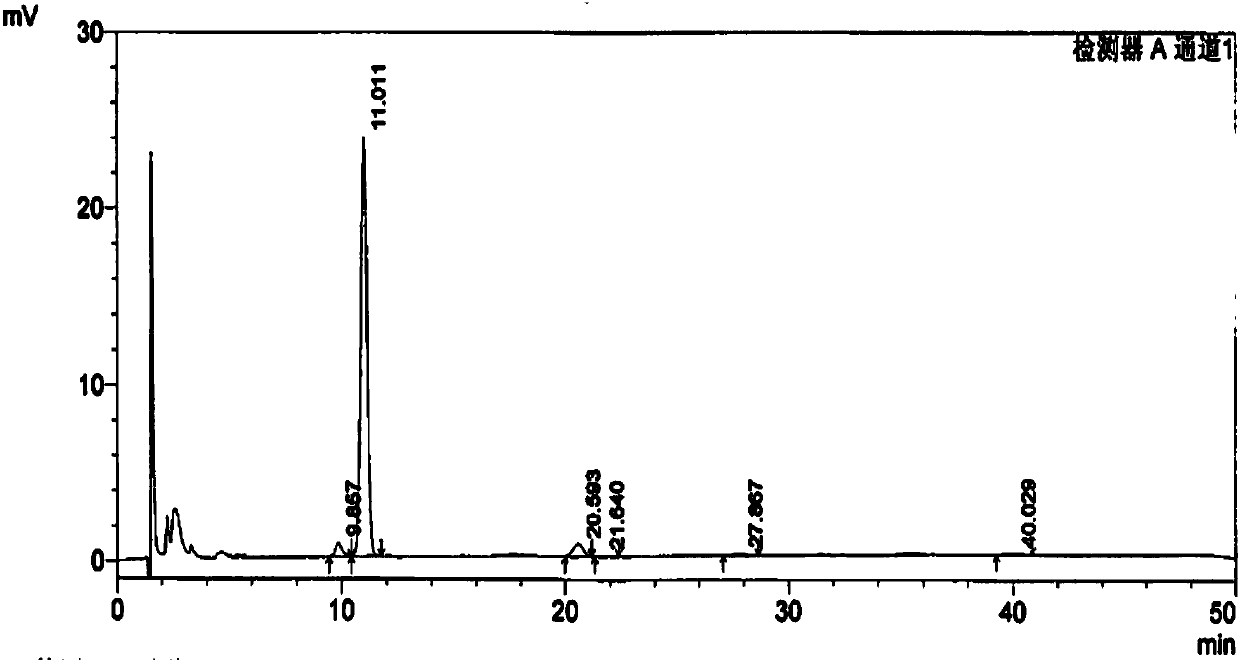
[0176] Except that the 20(R)-ginsenoside Rg3 whose batch number is 20160502 is weighed in step 2), the others are the same as in Example 1, and the measured batch number is 20160502 in the Shenyi Capsule crude drug 20(R)-ginsenoside Rg3 content It is 95.6%; Wherein the chromatogram under 203,210,225,270,320nm is identical with embodiment 1;
Embodiment 3
[0177] Example 3 Determination of the content of 20(R)-ginsenoside Rg3 of the Shenyi Capsule bulk drug whose batch number is 20160503
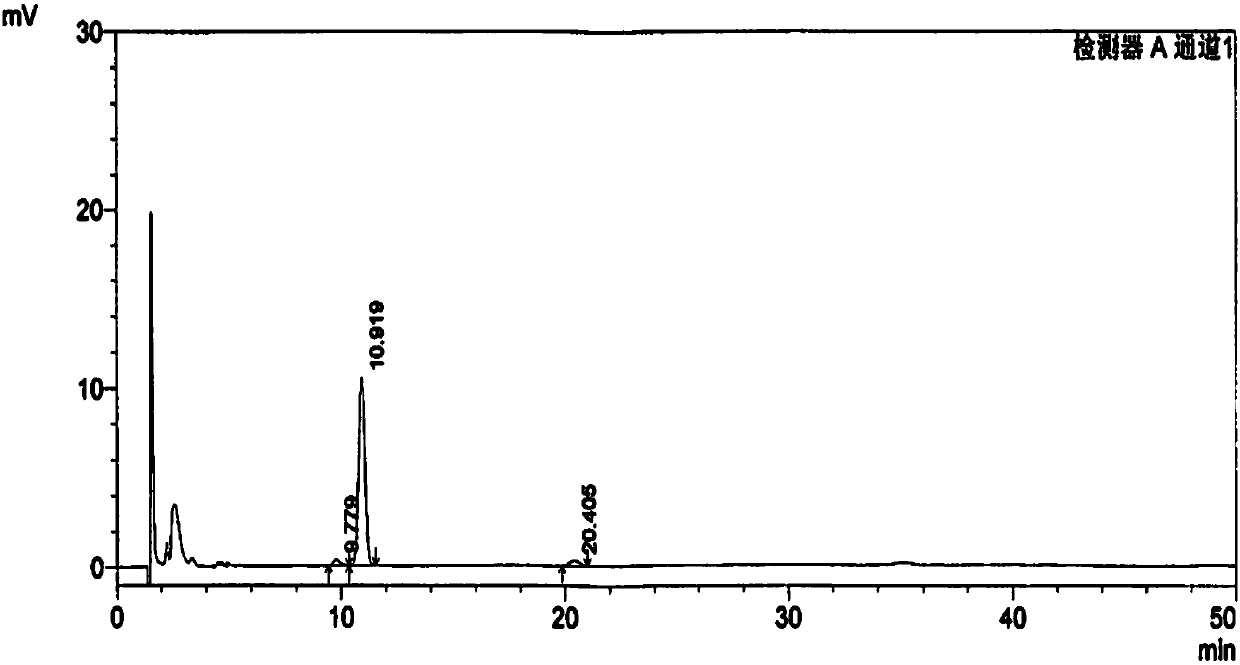
[0178] Except that the 20(R)-ginsenoside Rg3 whose batch number is 20160503 is weighed in step 2), the others are the same as in Example 1, and the measured batch number is 20160503 in the Shenyi Capsule raw material drug of 20(R)-ginsenoside Rg3 content It is 95.2%; wherein the chromatograms under 203, 210, 225, 270, 320nm are the same as in Example 1; the fingerprints after full-time multi-wavelength fusion are the same as in Example 1.
[0179] The fingerprint spectrogram obtained by the quality control method of the full-time multi-wavelength fusion fingerprint of Shenyi Capsule raw material medicine of the present invention can not only be used for fingerprint qualitative analysis of Shenyi Capsule raw material medicine, but also can be used for other preparations (such as 20 (R ) - fingerprint qualitative analysis of ginsenoside Rg3 gran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com