Non-obstructive azoospermia auxiliary diagnosis gene detection kit
A technology for azoospermia and auxiliary diagnosis, which can be used in the determination/examination of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc. It can solve the problems of difficult clinical diagnosis of non-obstructive azoospermia and achieve high specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The gene detection kit for auxiliary diagnosis of non-obstructive azoospermia provided by the present invention includes specific PCR primers, PCR buffer, dNTPs and DNA polymerase, wherein the specific PCR primers are the 69826819th position in the TEX11 gene for detecting male infertility A set of primers specific to the mutation site.
[0016] Wherein, the PCR buffer adopts 10 mmol / L Tris-Cl buffer, and the pH is 8.3-8.8.
[0017] DNA polymerase (DNA polymerase) is an important enzyme for cell replication DNA. DNA polymerase, using DNA as a replication template, is an enzyme that copies DNA from the 5' end to the 3' end. The main activity of DNA polymerase is to catalyze the synthesis of DNA and its complementary activities.
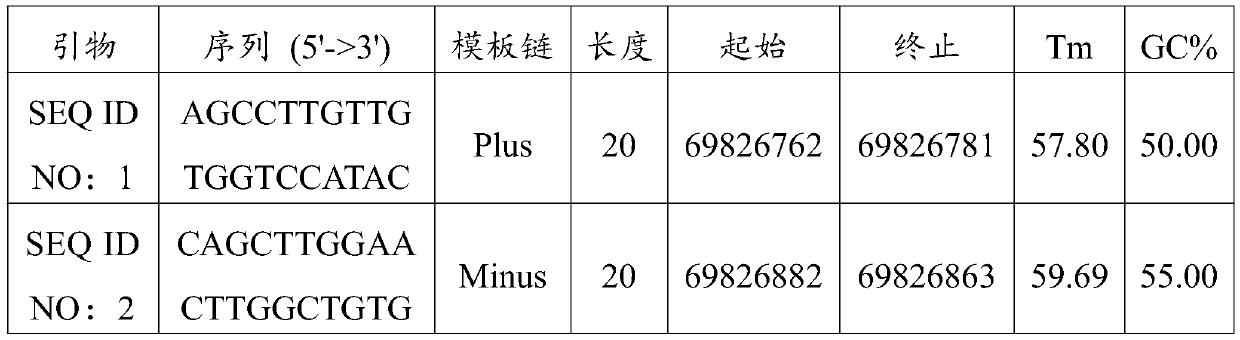
[0018] The upstream primer sequence of the specific PCR primer is shown in SEQ ID NO:1, and the downstream primer sequence is shown in SEQ ID NO:2. See Table 1 for sequence information.
[0019] Table 1
[0020]
[0021] Target gene name...
Embodiment 2
[0033] Example 2 The method of using the gene detection kit for auxiliary diagnosis of non-obstructive azoospermia
[0034] The gene detection kit for auxiliary diagnosis of non-obstructive azoospermia was used to detect the 69826819th mutation site in the TEX11 gene of male infertility. The specific operation process is as follows:
[0035] 1. PCR amplification
[0036] The PCR reaction conditions were: pre-denaturation at 95°C for 5min; denaturation at 95°C for 30s, annealing at 68°C for 2min30s, 30 cycles; extension at 72°C for 10min.
[0037] 2. Identification of PCR products: PCR products were identified by agarose electrophoresis analysis, and sent to Changchun Kumei Technology Co., Ltd. for sequencing after PCR identification.
[0038] 3. Observe the condition of the electrophoresis strip under ultraviolet light, and judge whether the sample contains a pathogenic mutation gene according to the condition of the electrophoresis strip.
[0039] Take the patient's blood a...
Embodiment 3
[0040] Embodiment 3 clinical trials
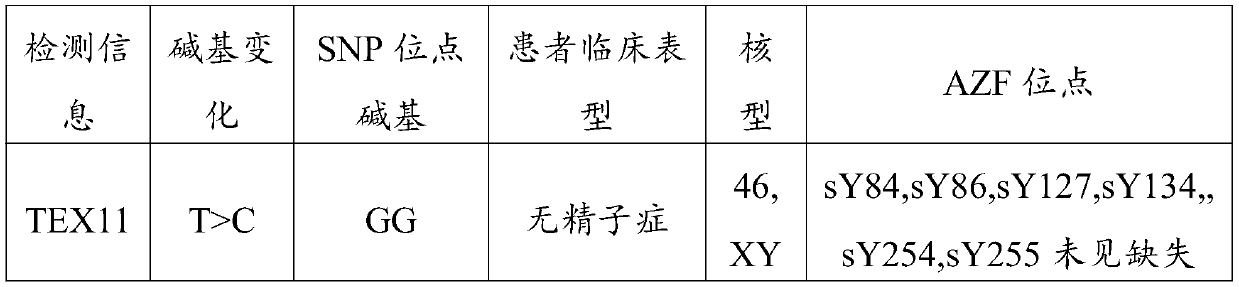
[0041] Screening of 30 patients with clinically confirmed non-obstructive azoospermia (the cause of these 30 patients is all caused by the mutation of the 69826819th gene in the TEX11 gene of male infertility, the position of the mutated gene is at 69826819, showing T >C homozygous mutation, the SNP of the two gene loci after the mutation is GG), using the non-obstructive azoospermia auxiliary diagnosis gene detection kit of embodiment 1, referring to the use method of embodiment 2 for these 30 patients Detection of genetic mutation sites. Observation of electrophoresis bands under ultraviolet light showed that there were no bands of 100 items, which indicated that the sample DNA template contained a mutant gene at the 69826819 mutation site, and the detection rate of gene mutation was 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com