Preparation method of cytotoxic T lymphocyte targeting to multiple KRAS mutant antigen epitopes of turmor
A technology for mutating antigens and lymphocytes, which is applied in the field of biotechnology development and application research, can solve the problems of prolonging the overall survival of patients, complicated operations, and low objective response rates, and achieves immune escape, broad-spectrum immune escape, and targeted highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Construction of a lentiviral expression vector carrying an antigen coding sequence and viral packaging:
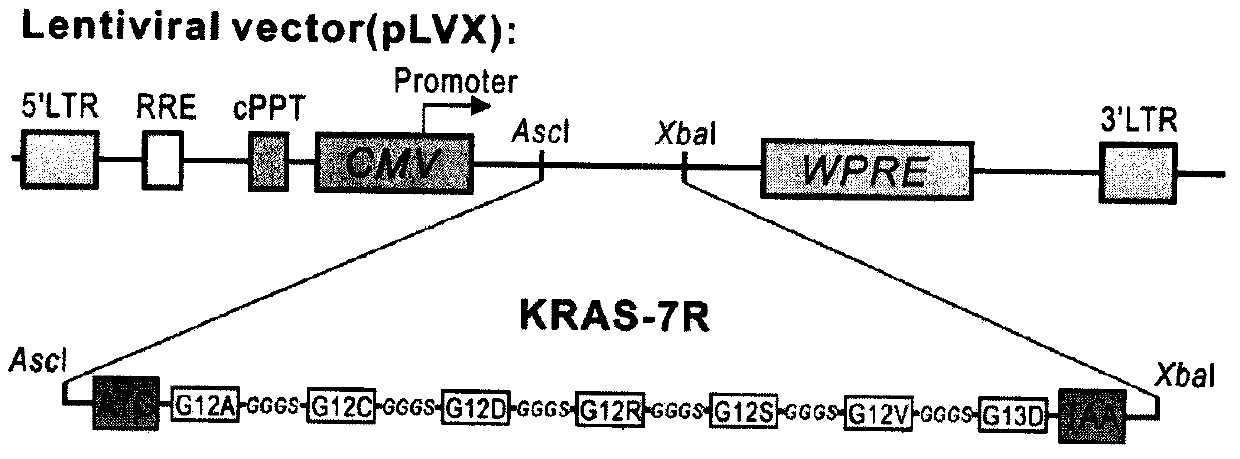
[0030] 1) Construction of lentiviral expression vector: The schematic diagram of the construction of lentiviral expression vector carrying the antigen coding sequence is shown in the attached figure 1 As shown, firstly, by using the Linker sequence, the epitope sequences (as shown in SEQ ID NO: 1-7) of the seven KRAS mutation sites (G12A, G12C, G12D, G12R, G12S, G12V and G13D) are connected in series Combining, and adding start and stop codons, creating a new artificial antigen coding sequence, as shown in SEQ ID NO: 9, the sequence size is 435bp; secondly, adding AscI and XbaI restriction enzyme cuts the site sequence and performs gene synthesis; finally, the synthesized antigen coding sequence fragment is double-digested by AscI and XbaI, and then connected into the lentiviral expression vector (pLVX) to construct the lentiviral expression vector (pLVX)...
Embodiment 2
[0032] Example 2. Induction culture and transfection of DC cells:
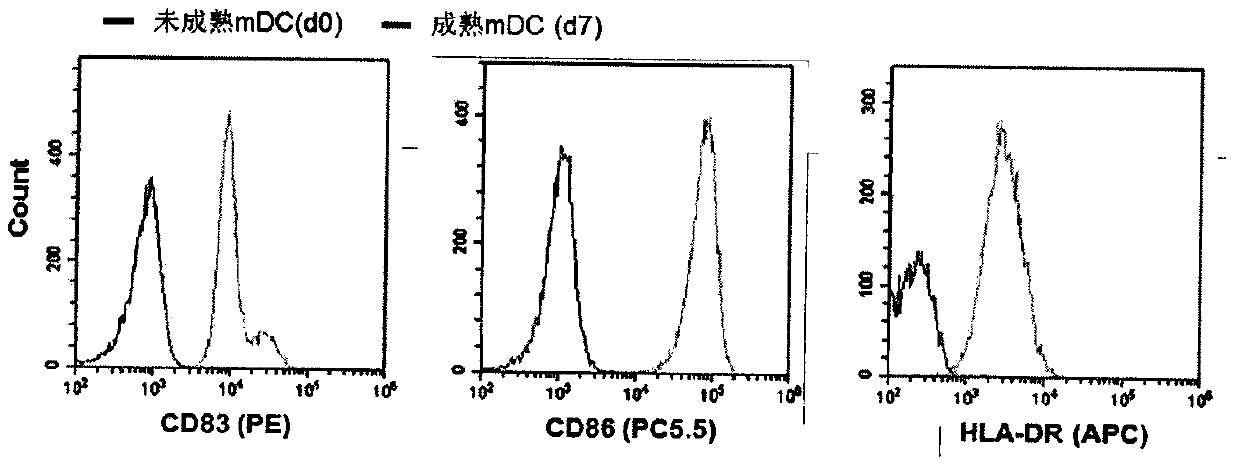
[0033] Collect peripheral blood, use Ficoll-Hypaque (polysucrose-diatrizoate meglumine) density gradient separation method to separate peripheral blood mononuclear cells PBMC, and use rhIL-4 (100ng / mL) and GM-CSF (1500U / mL) to stimulate and induce Culture DC cells. On the third day, immature DC cells were transfected with packaged and concentrated lentivirus particles, and the amount of lentivirus was calculated according to the optimal MOI value of the pre-experiment. After 24 hours of transfection, fresh DC medium was replaced and polyinosinic acid POLY (I:C) (10 μg / mL) was added to promote the expression of endogenous genes. Replenish fluid every other day, add tumor necrosis factor TNFα (10ng / mL) on the 6th day, and observe the maturation of the DC cells through a microscope on the 7th day, and at the same time detect the mature DC cells by flow cytometry and show that the markers CD83, CD86 and HLA- DR,...
Embodiment 3
[0034] Example 3. Preparation of antigen-specific CTL cells:
[0035] 1) Isolation and culture of CD8+T cells: PBMCs separated from peripheral blood by Ficoll, CD8+T cells were sorted by magnetic bead method. The sorted CD8+T cells were divided into 0.5-1×10 6 Inoculate in a T75 culture flask at a density of / mL, add cytokines IFN-γ (1000U / mL), rhIL-2 (1000U / mL) and autologous plasma (10%) to induce culture, and after 3 days of culture, add expansion in time Culture medium (1000U / mL rhIL-2). When the cells proliferated to an appropriate number, the cells were taken for flow cytometric analysis, as shown in the attached Figure 4 As shown, the expanded and cultured CD8+CTL cells have higher purity.
[0036] 2) Preparation of antigen-specific CTL cells: Co-culture antigen-loaded mature DCs and CTL cells at a ratio of 1:10 for 16 hours to sensitize the CTLs and generate specificity targeting seven major KRAS mutant epitopes
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com