Implantable drug-loaded device
An implantable, drug-loaded technology, applied in medical science, prosthesis, surgery, etc., can solve problems such as overactive effect, necrosis, tissue ulceration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
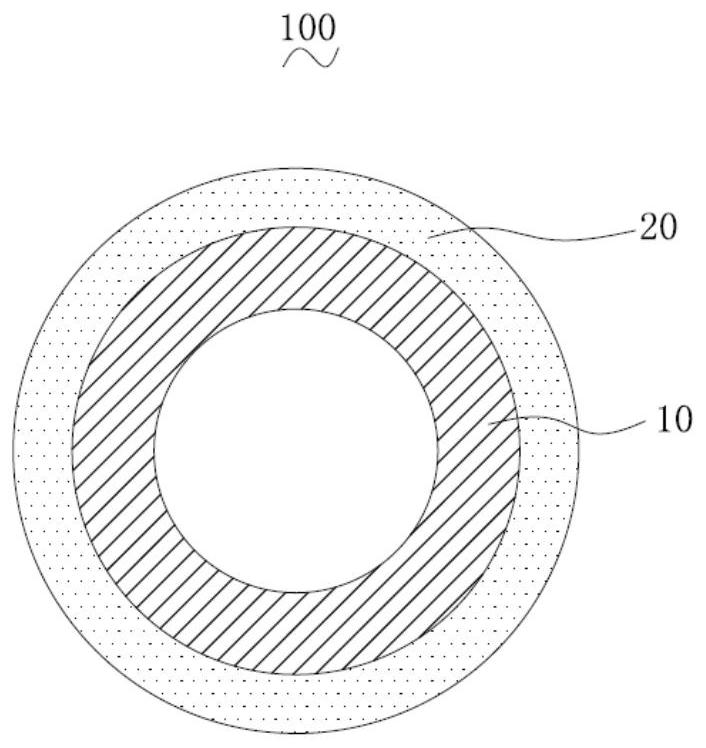
[0074] 34.6mm in surface area 2 The surface of the iron alloy vascular stent body is uniformly galvanized by electroplating to form a zinc coating on the surface of the iron alloy vascular stent body, and then the mixture of rapamycin and polylactic acid is uniformly sprayed on the surface of the zinc coating, and after drying, it is coated on the zinc coating A rapamycin-polylactic acid coating was formed on the surface to obtain the vascular stent of this embodiment. In this embodiment, the zinc coating is the zinc-containing part, and the ferroalloy stent body and the zinc coating form a zinc-containing matrix.
[0075] Through the aforementioned detection method, the content ρ of rapamycin in the zinc-containing matrix per unit area was measured to be 1.4 μg mm -2 The zinc content p corroded per unit time in the zinc-containing substrate per unit area in 8.3vol.% superior pure acetic acid aqueous solution at 25 ° C is 0.065 μg mm -2 min -1 , 15p+ρ=2.375.
[0076] The s...
Embodiment 2
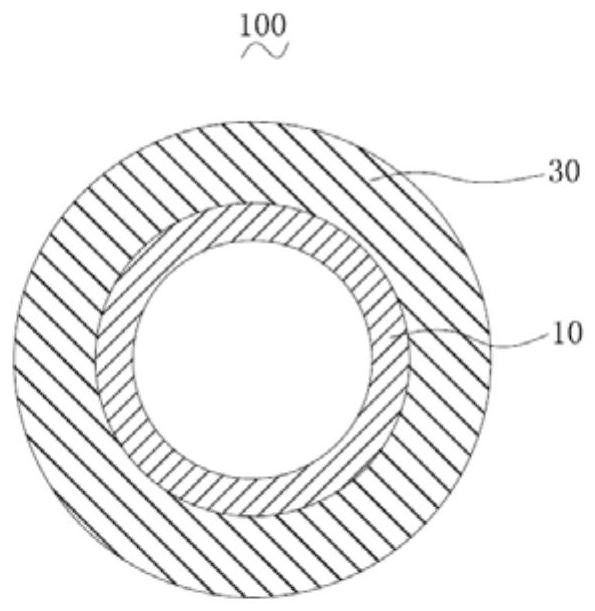
[0078] will have a surface area of 34.6mm 2 Half of the pure iron vascular stent body along the length direction is immersed in the galvanizing solution, and the electroplating method is used to uniformly galvanize to form a zinc coating on the half surface of the pure iron vascular stent body, and then the galvanized pure iron vascular stent The main body is placed in a vacuum furnace for heat treatment. The furnace cavity pressure is 20 Pa, the heat treatment temperature is 300 ° C, and the heat treatment time is 3 hours. After the mixture of lactic acid is dried, a rapamycin-polylactic acid coating is formed on the surface of the stent body to obtain the vascular stent of this embodiment. In this embodiment, a zinc-iron alloy layer covering the surface of the pure iron vascular stent body is formed after heat treatment, the zinc-iron alloy layer is the zinc-containing part, and the pure iron vascular stent body and the zinc-iron alloy layer form a zinc-containing matrix. ...
Embodiment 3
[0082] 34.6mm in surface area 2 The surface of the iron alloy vascular stent body is uniformly galvanized by electroplating to form a zinc coating on the surface of the iron alloy vascular stent body, and finally the mixture of rapamycin and polylactic acid is evenly sprayed on the surface of the zinc coating, and after drying, it is coated on zinc A rapamycin-polylactic acid coating was formed on the surface of the coating to obtain the vascular stent of this embodiment. In this embodiment, the zinc coating is the zinc-containing part, and the ferroalloy stent body and the zinc coating form a zinc-containing matrix.
[0083] Through the aforementioned detection method, the content ρ of rapamycin in the zinc-containing matrix per unit area was measured to be 0.5 μg mm -2 The zinc content p corroded per unit time of the zinc-containing substrate per unit area in 8.3vol.% superior pure acetic acid aqueous solution at 25 ° C is 0.14 μg mm -2 min -1 , 15p+ρ=2.6.
[0084] The s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com