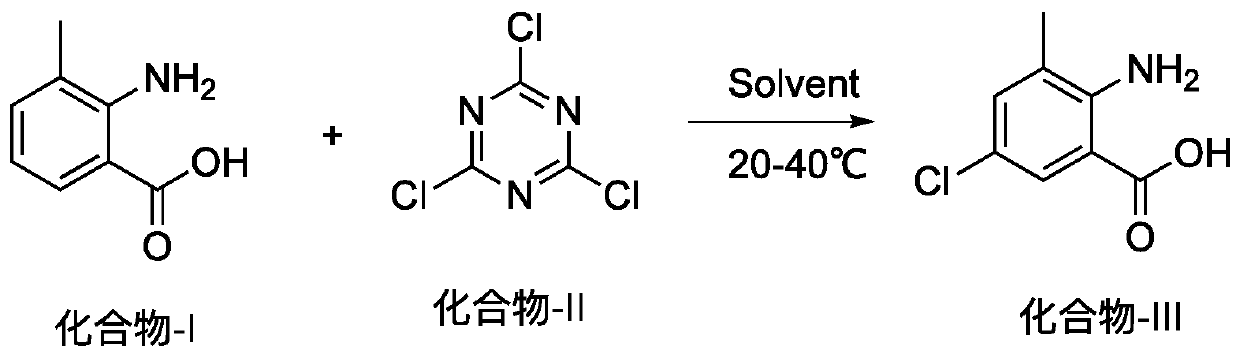
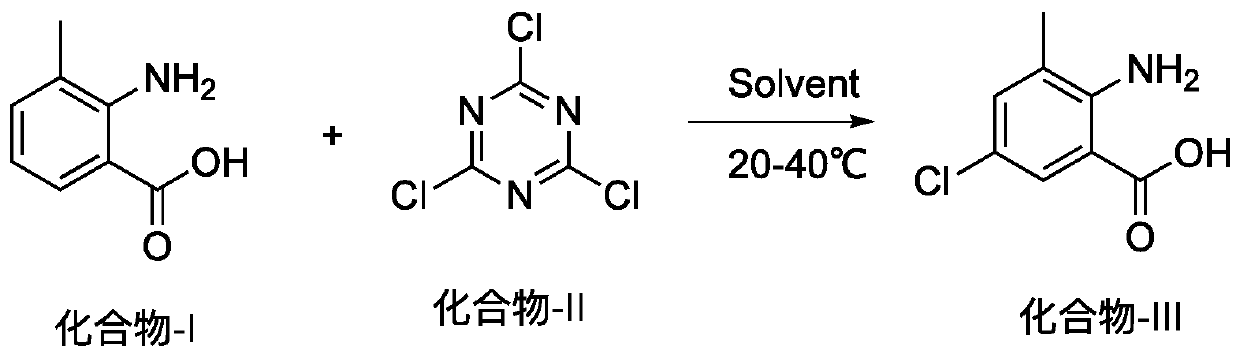
Method for preparing 2-amino-5-chloro-3-methylbenzoic acid
A technology of toluic acid and amino, which is applied in chemical instruments and methods, cyanide reaction preparation, organic compound preparation, etc., can solve the problems of high price and difficult post-processing, and achieve high overall yield, simple raw materials, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] At room temperature, in a 1000ml round bottom flask, add isatoic anhydride (60g, 396.92mmol), dichloroethane 180ml, at 25-30°C, slowly add cyanuric chloride (25.62g, 138.92mmol) in batches. After the addition, continue to stir for about 10 hours. The liquid phase detection of indigo raw materials is less than 1%, and the temperature is lowered to below 5°C. Suction filter, wash the solid twice with 50°C water (150ml), and dry it. Transfer to a reaction bottle, add anhydrous methanol (250ml), heat until the system dissolves, cool to below 5°C, filter with suction, and dry to obtain 62.6g of off-white solid with a yield of 85% and a liquid phase detection purity of 98.5 %.
[0013] 1H NMR (DMSO-d6) 7.55 (s, IH), 7.22 (s, IH), δ 2.11 (s, 3H).
Embodiment 2
[0015] At room temperature, add isatoic anhydride (5kg, 33.08mol) and 30L dichloroethane into a 50L reactor, and slowly add cyanuric chloride (2.13kg, 11.58mol) in batches at 25-30°C. After the addition, continue to stir for about 10 hours. The liquid phase detection of indigo raw materials is less than 1%, and the temperature is lowered to below 5°C. Suction filter, wash the solid twice with 50°C water (15L), and drain it. Transfer to a reaction bottle, add anhydrous methanol (20L), heat until the system dissolves, cool to below 5°C, filter with suction, and dry to obtain 5.1kg of off-white solid with a yield of 83% and a liquid phase detection purity of 98.8 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com