Vector combination for quick gene editing of halomonas sp. and application of vector combination
A Halomonas and gene editing technology, applied in the field of genetic engineering, can solve the problems of cost, prolongation, and impossibility of knockout, etc., and achieve the effect of rapid gene editing, simplified steps, and reduced time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
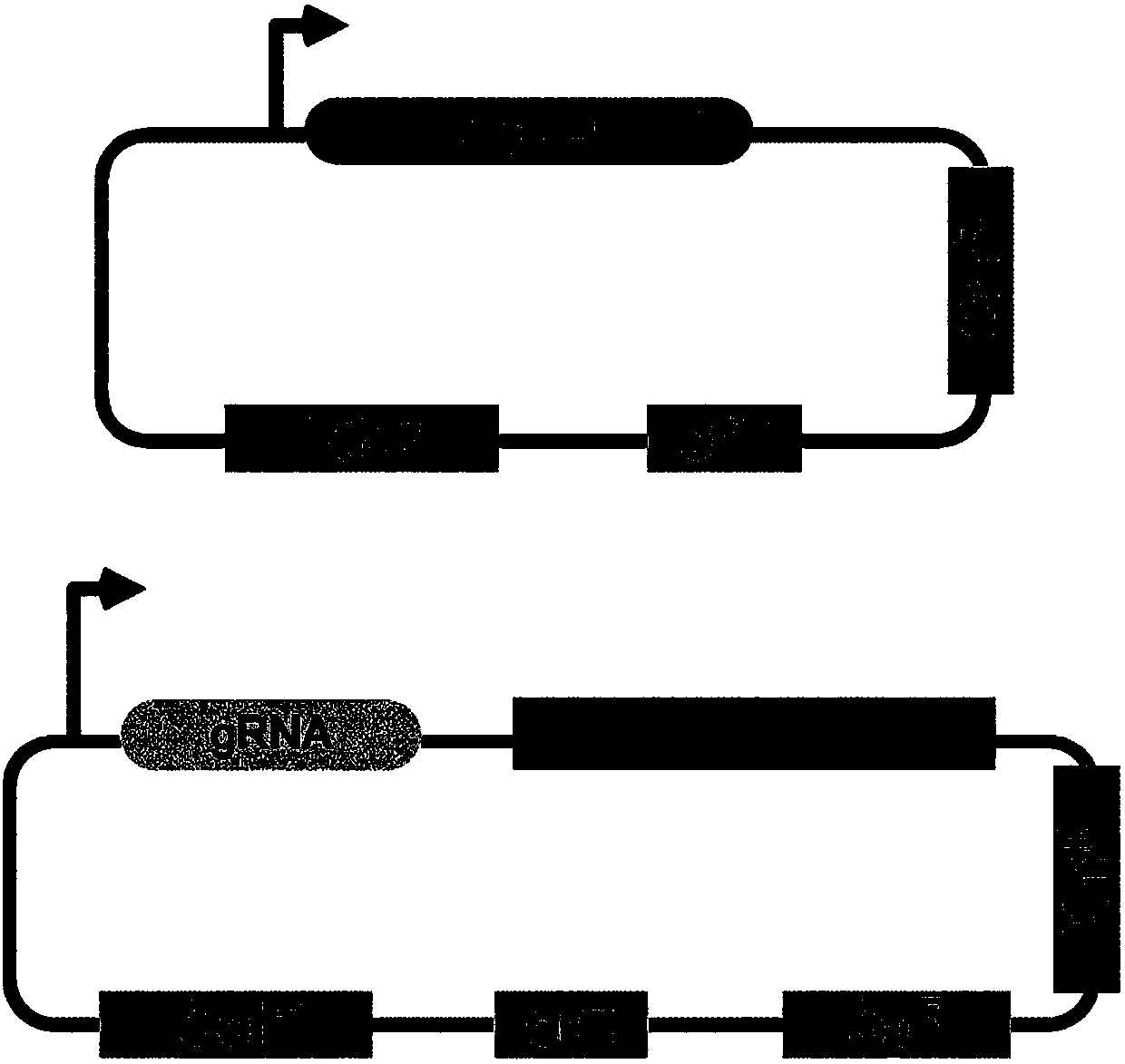
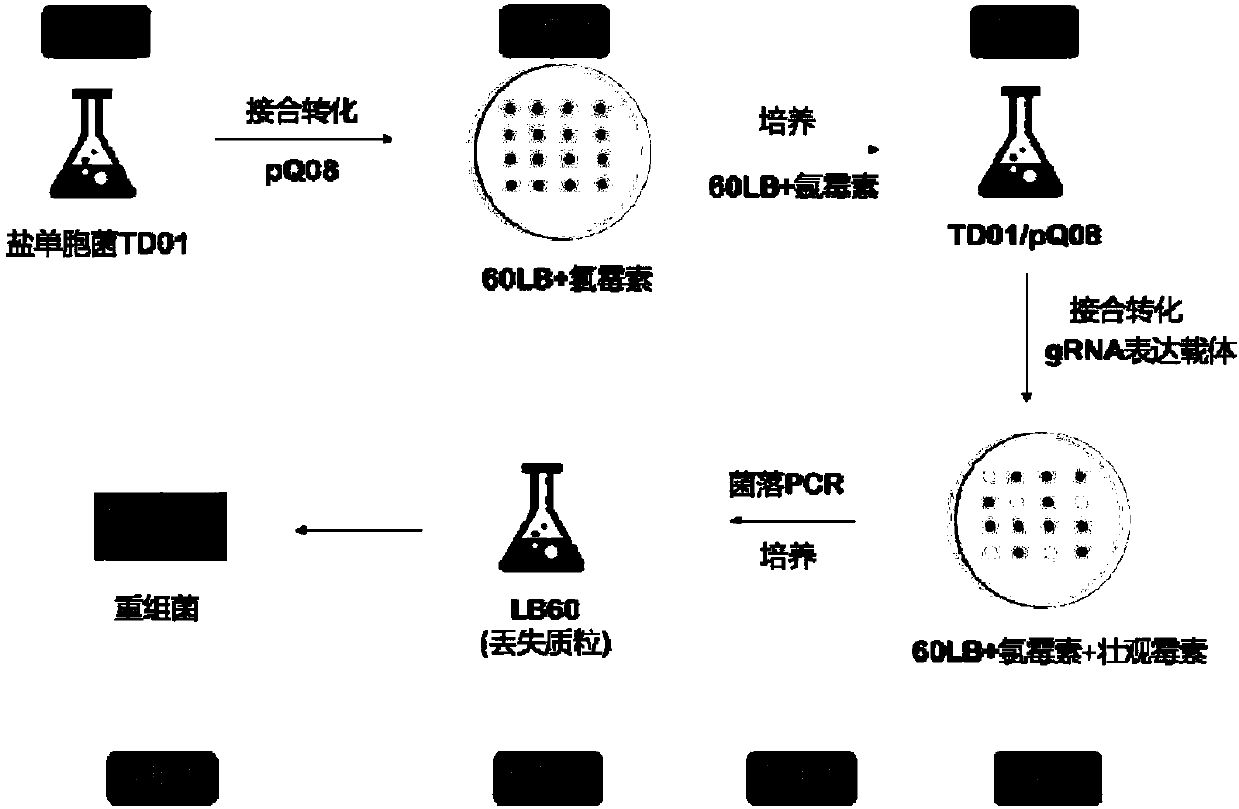
[0048] Example 1: Construction of a vector for gene editing of Halomonas TD01 based on CRSIPR / Cas9
[0049] Using primers to pCas plasmid (Jiang, Y., Chen, B., Duan, C., Sun, B., Yang, J., Yang, S., 2015. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9system .Appl.Environ.Microbiol.81, 2506-2514) was amplified as a template to obtain a DNA fragment containing a promoter, a cas9 gene and a terminator, and was connected into pSEVA321 (Silva-Rocha, R., de Lorenzo, V., 2013. The Standard European Vector Architecture (SEVA): a coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 41, 666–675.) Between the XmaI and SacII sites, the cas9 expression vector pQ08 was obtained. The primers used are as follows:
[0050]
[0051]
[0052] The resulting vector sequence is:
[0053]
[0054] Primers were used to amplify pSEVA341 (Silva-Rocha, R., de Lorenzo, V., 2013. The Standard European Vector Architecture (SEVA...
Embodiment 2
[0063] Example 2: Knockout of the phaC gene of Halomonas TD01 based on CRISPR / Cas9
[0064] In the phaC gene sequence of Halomonas TD01, gataacattgccgtcacccc (SEQ ID NO: 13) was selected as gRNA, and the corresponding PAM sequence was AGG. Use primers AGCCGAAGACTGTAGTGATAACATTGCCGTCACCCC (SEQ ID NO: 14) and TACAGAAGACAGAAACGGGGTGACGGCAATG (SEQ ID NO: 15) to perform template-free PCR to obtain a 52bp fragment containing gRNA, which is connected between the BbsI sites of pHALORNA by the Golden Gate ligation method. At the same time, using the genome of Halomonas TD01 (preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee, the preservation number is CGMCCNo.4353) as a template, primers TAAAGGTCTCAGCGGGAAGCATGGAAAGTGCAGCT (SEQ ID NO: 16) and TTCTCACGCAGTGCAGCGCATGACTTCGGG (SEQ ID NO : 17) Amplify the upstream sequence of the homology arm of 522bp, and amplify the downstream sequence of the homology arm of 520bp with primers ...
Embodiment 3
[0079] Example 3: Inserting exogenous genes into the genome of Halomonas TD01 based on CRISPR / Cas9
[0080]In the genome sequence of Halomonas TD01, ttcacctagctagatgagac (SEQ ID NO: 30) was selected as the gRNA of the insertion site, and the corresponding PAM sequence was AGG. Use primers AGCCGAAGACTGTAGTTTCACCTAGCTAGATGAGAC (SEQ ID NO: 31) and TACAGAAGACAGAAACGTCTCATCTAGCTAG (SEQ ID NO: 32) to perform template-free PCR to obtain a 52bp fragment containing gRNA, which is connected between the BbsI sites of pHALORNA by the Golden Gate ligation method. At the same time, using the genome of Halomonas TD01 as a template, use primers to amplify the upstream sequence of the homology arm, and use another pair of primers to amplify the downstream sequence of the homology arm; using the genome of Halomonas TD01 as a template, use The primers were amplified to obtain a fragment containing the porin promoter; G.Q.,2005.Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)production inrecombinan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com