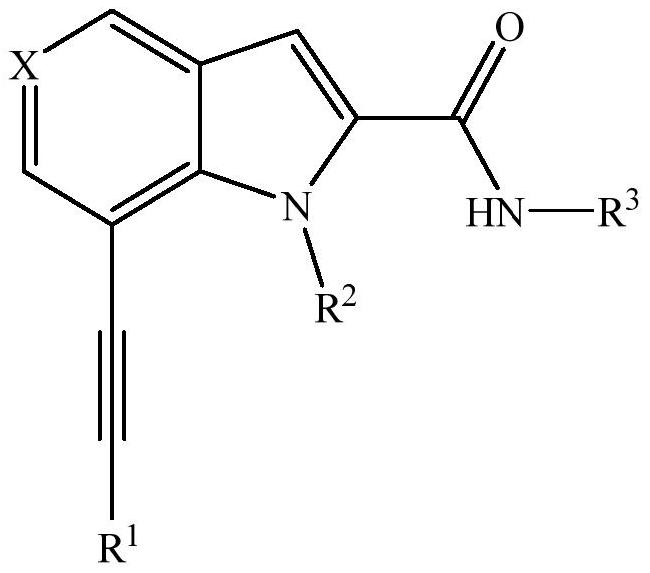
A kind of alkyne compound, preparation method and application thereof
A technology of compounds and enantiomers, applied in the application field of drug preparation, can solve the problems of side effects limiting the clinical application of drugs, no inhibitory activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] 1‐methyl‐N‐(4‐((4‐methylpiperazine‐1‐)methyl)phenyl)‐7‐(pyridine‐3‐ethynyl)‐1H‐indole‐2‐carboxamide preparation
[0182] Step 1) Preparation of 7-bromo-1-methyl-N-(4-((4-methylpiperazine-1-)methyl)phenyl)-1H-indole-2-formamide
[0183]
[0184] 7-bromo-1-methyl-1H-indole-2-carboxylic acid (2.5g, 10mmol), 4-((4-methylpiperazine-1-)methyl)amine (2.0g, 10mmol) and 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) (3.8g, 10mmol) dissolved in N,N-dimethylformaldehyde Add diethylisopropylamine (1.65mL, 10mmol) to the amide, stir until the reaction is complete, extract with ethyl acetate and water, concentrate the organic phase, and perform column chromatography to obtain 3.1 g of the target compound with a yield of 70%. 1 H NMR (400MHz, DMSO-d 6 )δ10.48(s,1H),7.76–7.68(m,3H),7.53–7.49(m,1H),7.27(d,J=8.4Hz,2H),7.23(s,1H),7.04(t ,J=7.7,7.7Hz,1H),4.28(s,3H),3.43(s,2H),2.48–2.27(m,8H),2.19(s,3H); MS:441[M+H] + .
[0185] Step 2) 1‐methyl‐N‐(...
Embodiment 2
[0192] 1‐methyl‐N‐(4‐((4‐methylpiperazine‐1‐)methyl)‐3‐(trifluoromethyl)phenyl)‐7‐(pyridine‐3‐ethynyl)‐1H ‐Indole‐2‐carboxamide preparation
[0193] Step 1) 7‐bromo‐1‐methyl‐N‐(4‐((4‐methylpiperazine‐1‐)methyl)‐3‐(trifluoromethyl)phenyl)‐1H‐indole‐ Preparation of 2‐formamide
[0194]
[0195] 7-Bromo-1-methyl-1H-indole-2-carboxylic acid (2.5g, 10mmol), 4-((4-methylpiperazine-1-)methyl)-3-(trifluoromethyl ) aniline (2.73g, 10mmol) and 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU) (3.8g, 10mmol) Add diethylisopropylamine (1.65mL, 10mmol) to N,N-dimethylformamide, stir until the reaction is complete, extract with ethyl acetate and water, concentrate the organic phase, and column chromatography to obtain 3.6 grams of product , yield 70%. 1 H NMR (400MHz, DMSO-d 6 )δ10.74(s,1H),8.22(s,1H),8.05–7.99(m,1H),7.77–7.69(m,2H),7.53(d,J=7.5Hz,1H),7.30(s ,1H),7.05(t,J=7.7,7.7Hz,1H),4.30(s,3H),3.57(s,2H),2.47–2.23(m,8H),2.17(s,3H); MS: 509[M+H] ...
Embodiment 3
[0203] N‐(4‐((4‐methylpiperazine‐1‐)methyl)‐3‐(trifluoromethyl)phenyl)‐7‐(pyrimidine‐5‐ethynyl)‐1H‐indole‐2 ‐ Formamide preparation
[0204] Step 1) of 7‐bromo‐N‐(4‐((4‐methylpiperazine‐1‐)methyl)‐3‐(trifluoromethyl)phenyl)‐1H‐indole‐2‐carboxamide preparation
[0205]
[0206]7-Bromo-1H-indole-2-carboxylic acid (1) (2.4g, 10mmol), 4-((4-methylpiperazine-1-)methyl)-3-(trifluoromethyl)aniline N , into N-dimethylformamide, add diethylisopropylamine (1.65mL, 10mmol), stir until the reaction is complete, extract with ethyl acetate and water, concentrate the organic phase, and column chromatography to obtain 3.5 grams of product, yield rate of 70%. 1 H NMR (400MHz, DMSO-d 6 )δ11.67(s,1H),10.65(s,1H),8.23(d,J=2.1Hz,1H),8.10–8.02(m,1H),7.80–7.70(m,2H),7.55–7.45 (m,2H),7.06(t,J=7.8Hz,1H),3.58(s,2H),2.49–2.24(m,8H),2.18(s,3H); MS:495[M+H] + .
[0207] Step 2) N‐(4‐((4‐methylpiperazine‐1‐)methyl)‐3‐(trifluoromethyl)phenyl)‐7‐((trimethylsilyl)ethynyl)‐ Preparation of 1H‐indole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com