Selenium cyanide compound and application thereof
A compound, haloacyl halide technology, applied in the field of selenocyanide and its preparation, can solve the problems of inability to prevent cancer cells from spreading to other organs, limited anti-cancer spectrum, and further improvement of anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] In the preparation method of the present invention, sodium bicarbonate is preferably used as a catalyst in step (i).
[0025] An exemplary reaction scheme is as follows:
[0026]
[0027] The preparation method of the invention is simple, the yield is high, and the compound of formula I can be easily prepared.
[0028] The present invention also provides the use of a compound of formula I or a pharmaceutically acceptable salt thereof in the preparation of a medicament for preventing and / or treating tumors; preferably, the tumors are selected from colon cancer, breast cancer, prostate cancer, cervical cancer, Gastric cancer, liver cancer, lung cancer and leukemia; particularly preferably, the tumor is selected from gastric cancer, lung cancer, liver cancer and leukemia.
[0029] In another aspect, the present invention provides a pharmaceutical composition comprising a compound of formula I or a pharmaceutically acceptable salt thereof, and optionally pharmaceuticall...
specific Embodiment approach
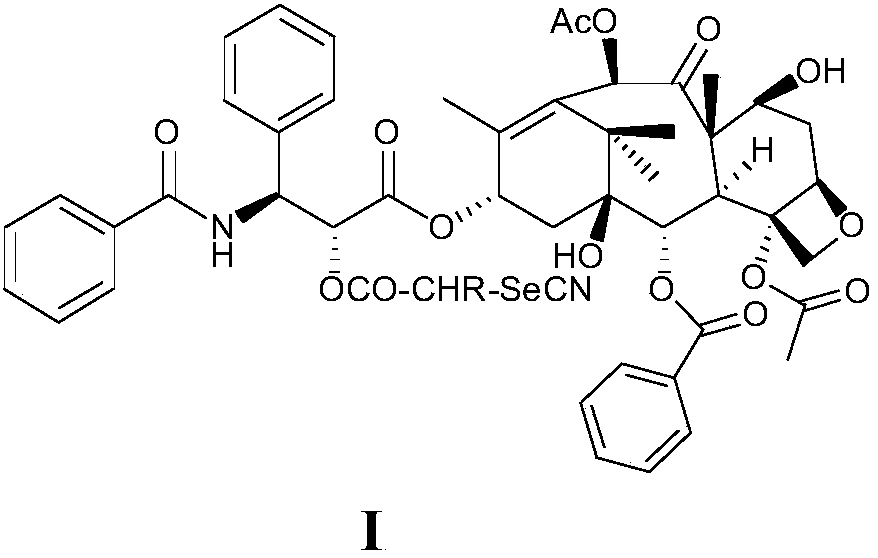
[0030] The present invention provides the compound described in following formula I
[0031]
[0032] or a pharmaceutically acceptable salt thereof,
[0033] wherein R is a linear or branched alkyl group of 1 to 6 carbon atoms or hydrogen. Preferably, R is a linear or branched alkyl group of 1 to 4 carbon atoms or hydrogen; more preferably, R is selected from methyl, ethyl, n-propyl, isopropyl, n-butyl, iso Butyl, tert-butyl and hydrogen; in a particularly preferred embodiment, R is selected from methyl, ethyl, n-propyl, isopropyl and hydrogen; in a particularly preferred embodiment, R is selected from methyl radical, ethyl and hydrogen; in a more preferred embodiment, R is selected from methyl and hydrogen.
[0034] The pharmaceutical composition of the present invention comprises the compound of formula I of the present invention or a pharmaceutically acceptable salt thereof, and optional pharmaceutically acceptable excipients and / or carriers. In the pharmaceutical com...
preparation Embodiment 1
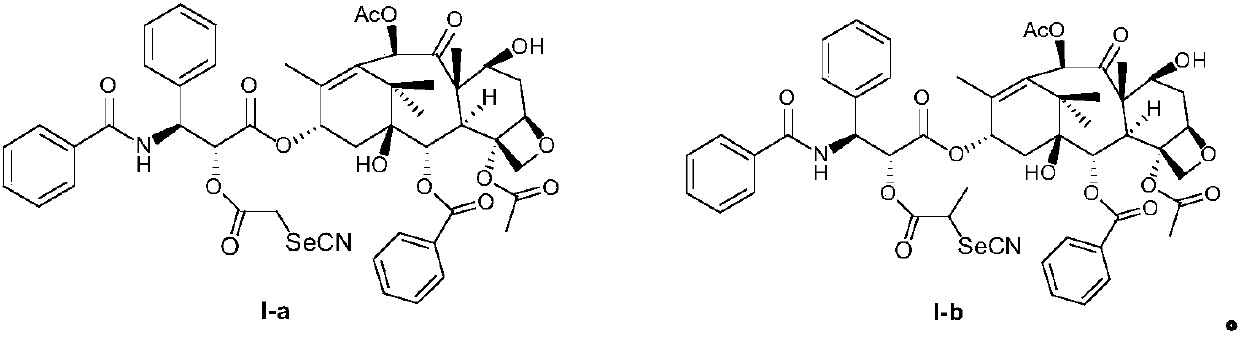
[0053] Preparation Example 1: Synthesis of Compound I-a
[0054] The reaction formula is as follows:
[0055]
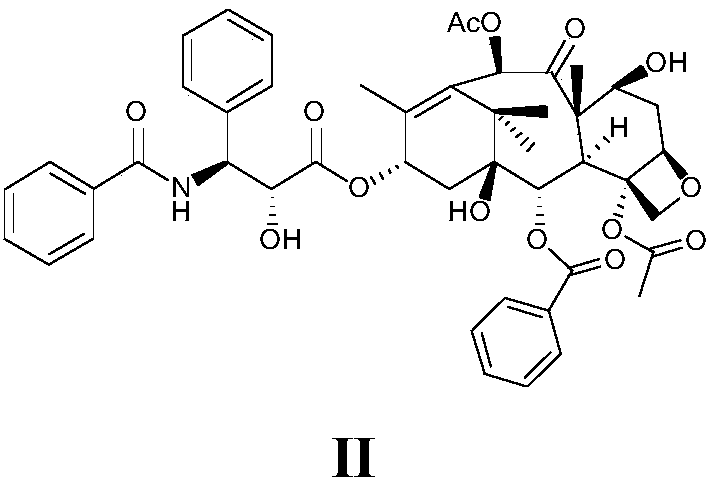
[0056] In a three-necked flask, paclitaxel (200 mg, 0.23 mmol) was dissolved in a mixed solvent of dichloromethane and water (CH 2 Cl 2 (V): H 2 O(V)=1:1), sodium bicarbonate (42mg, 0.2mmol) was added, bromoacetyl bromide (56mg, 0.28mmol) was added at room temperature, stirred for 1 hour, TLC detected that the reaction was complete. The reaction solution was washed twice with 1N HCl (35ml), and 1% NaHCO 3 (35ml) Na after washing twice 2 SO 3 dry. After the solvent was evaporated to dryness, the solid was dissolved by heating with EtOAc and crystallized on standing. Filtration gave a light brown solid, compound II (191 mg, 71% yield). Dissolve directly in anhydrous acetonitrile, add potassium selenocyanate 145 (33 mg, 0.23 mmol) in batches, and stir at room temperature for 16 hours. After the reaction solution was concentrated, it was dropped into 150ml of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com