Thermally-activated delayed fluorescent material, preparation method thereof, and organic light-emitting diode device
A technology of heat-activated delayed and fluorescent materials, applied in the direction of luminescent materials, electrical solid devices, chemical instruments and methods, etc., can solve the problems of lack of heavy metal Ir complexes, etc., achieve high device efficiency and improve luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The synthetic route of target compound 1 is as follows:
[0045]
[0046] 9,10-dihydro-9,9-dimethylacridine (2.51g, 12mmol) was added into a 100mL two-necked flask, and then sodium hydride NaH (0.48g, 12mmol) was added in the glove box. Add 40 mL of tetrahydrofuran (THF) that had been dehydrated and deoxygenated beforehand, react at 60°C for 2 hours, then add raw material 1 (5mmol, 1.35g), and react at 60°C for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 2.0 g of compound 1 as orange-red powder, yield 62%.
[0047] 1HNMR (300 MHz, CD 2 Cl 2 ,δ): 8.74 (s, 4H), 7.19-7.14 (m, 12H), 6.98-6.93 (m, 4H), 1.69 (s, 12H).
[0048] MS(EI)m / z:[M] + calcd for C 42 h 32 N 8 , 648.27; found, 648.18.
Embodiment 2
[0050] The synthetic route of target compound 2 is as follows:
[0051]
[0052] Add phenoxazine (2.20g, 12mmol) into a 100mL two-necked bottle, then add NaH (0.48g, 12mmol) into the glove box, inject 40mL of tetrahydrofuran that has been dehydrated and deoxygenated beforehand under an argon atmosphere, and put it at 60°C After reacting for 2 hours, raw material 1 (5 mmol, 1.35 g) was added, and reacted at 60° C. for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 1.9 g of compound 2 as a red powder, yield 64%.
[0053] 1 H NMR (300MHz, CD 2 Cl 2 ,δ): 8.74 (s, 4H), 7.14-7.06 (m, 4H), 7.01-6.95 (m, 12H).
[0054] MS(EI)m / z:[M] + calcd for C 36 h 20 N 8 o 2 , 596.17; found, 596.16.
Embodiment 3
[0056] The synthetic route of target compound 3 is as follows:
[0057]
[0058] Add phenothiazine (2.39g, 12mmol) into a 100mL two-necked flask, then add NaH (0.48g, 12mmol) into the glove box, inject 40mL of tetrahydrofuran that has been dehydrated and deoxygenated beforehand under an argon atmosphere, and put it at 60°C After reacting for 2 hours, raw material 1 (5 mmol, 1.35 g) was added, and reacted at 60° C. for 24 hours. Cool to room temperature, pour the reaction solution into 200mL ice water, extract three times with dichloromethane, combine the organic phases, spin into silica gel, and separate and purify by column chromatography (dichloromethane:n-hexane, v:v, 3:2) to obtain 1.5 g of compound 3 as dark red powder, yield 48%.
[0059] 1 H NMR (300MHz, CD 2 Cl 2 ,δ): 8.74 (s, 4H), 7.21-7.13 (m, 12H), 6.97-6.88 (m, 4H).
[0060] MS(EI)m / z:[M] + calcd for C 36 h 20 N 8 S 2 , 628.13; found, 628.10.
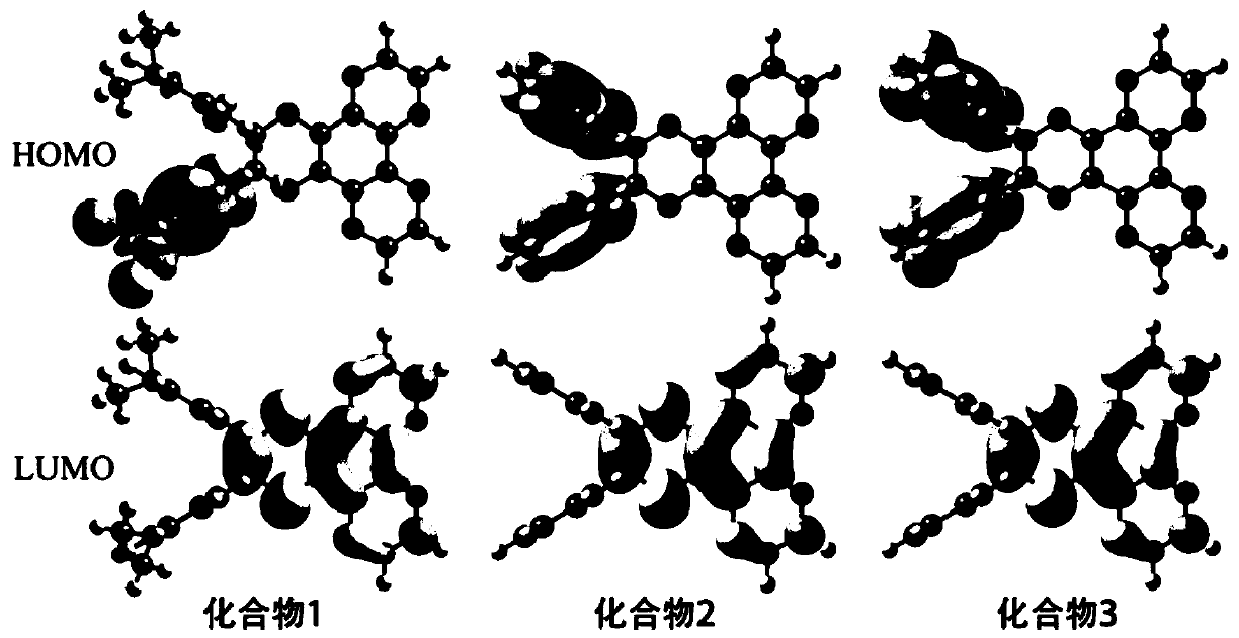
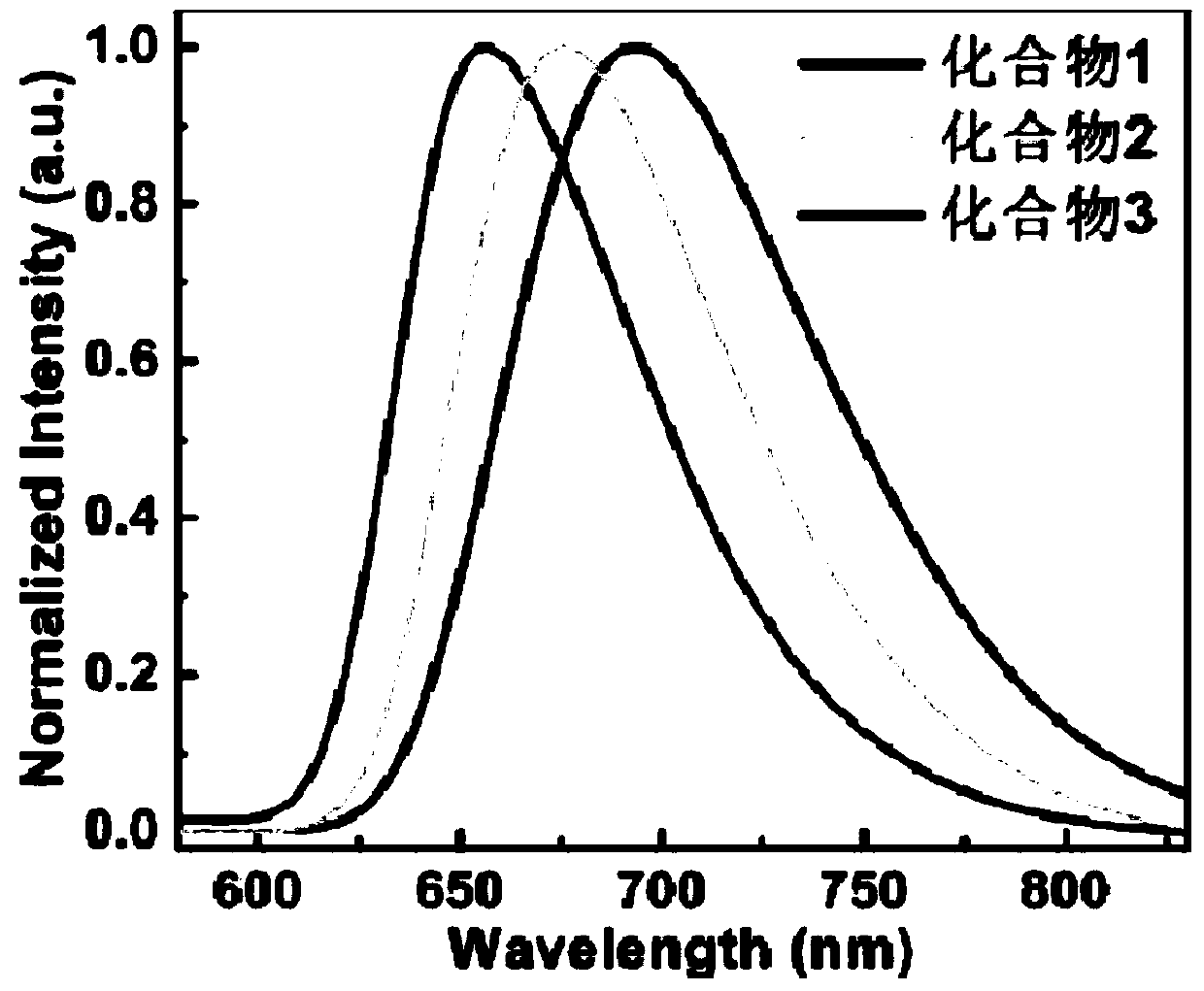
[0061] figure 1 shows the orbital arrangement of compoun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com