Ionic liquid functionalized acid orange for organic solvent and preparation method thereof
A technology of ionic liquid and organic solvent, which is applied in the field of ionic liquid functionalized acid orange dye and acid-base indicator and its preparation, achieving the effect of mild reaction conditions and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of ethyltriphenylphosphonium bromide:
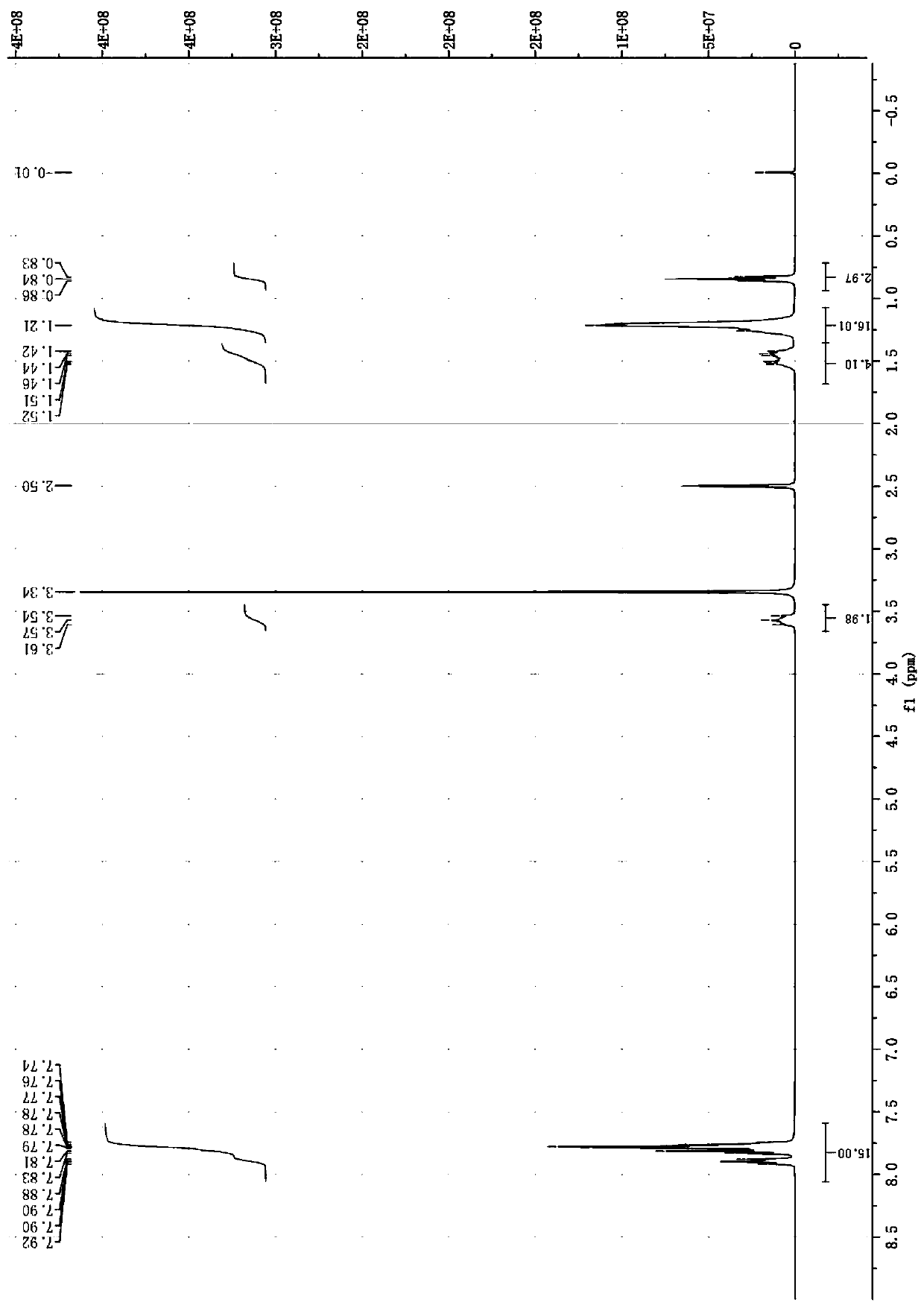
[0033] Add 1.31 g (5.0 mmol) of triphenylphosphine and 20 mL of dry benzene into a 100 mL three-necked flask, stir and dissolve at room temperature. Then, 0.60 g (5.5 mmol) of 1-bromoethane was slowly added dropwise with stirring. After the addition was completed, the mixture was stirred and reacted at 40°C for 48 hours. After the reaction, cool to room temperature, filter with suction, and wash repeatedly 3 times to obtain a white solid, which is vacuum-dried to obtain 1.82 g of bromobutyltriphenylphosphonium as a white powder solid, with a yield of 89.2%.
[0034] 1 H NMR (400MHz, DMSO-d6) (ppm): δ=7.75-7.93 (m, 15H, H on the benzene ring), 3.34-3.66 (t, 2H, H on the methylene connected to P), 1.17-1.27 (t, 3H, H on methyl in ethyl).
[0035] Synthesis of acid orange silver salt:
[0036] Under nitrogen protection, 0.84 g (2.4 mmol) of Acid Orange II was dissolved in a small amount of deionized water, acidified by...
Embodiment 2
[0042] Synthesis of butyltriphenylphosphonium bromide:
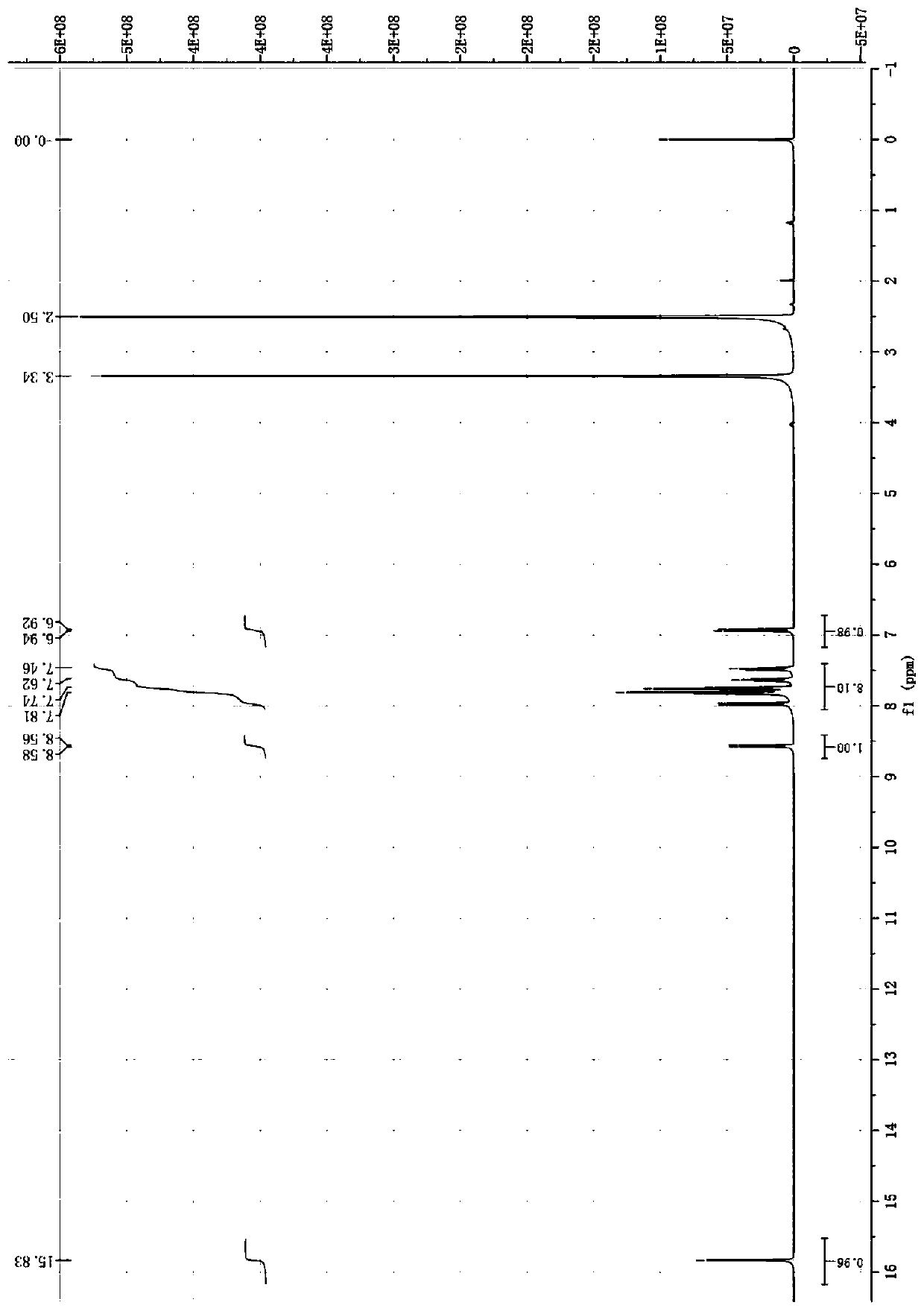
[0043] Add 1.31 g (5.0 mmol) of triphenylphosphine and 20 mL of dry toluene into a 100 mL three-necked flask, stir and dissolve at room temperature. Then, 0.69 g (5.0 mmol) of 1-bromobutane was slowly added dropwise with stirring. After the addition was complete, the mixture was stirred and reacted at 100°C for 48 hours. After the reaction, cool to room temperature, filter with suction, and wash repeatedly 3 times to obtain a white solid, which is vacuum-dried to obtain 1.85 g of bromobutyltriphenylphosphonium as a white powder solid, with a yield of 92.5%.
[0044] 1 H NMR (400MHz, DMSO-d6) (ppm): δ=7.75-7.92 (m, 15H, H on the benzene ring), 3.34-3.62 (t, 2H, H on the methylene connected to P), 1.45-1.52 (m, 4H, the H on the methylene in the butyl group except the methylene directly connected to P), 0.87-0.91 (t, 3H, the H on the methyl group in the butyl group).
[0045] Synthesis of acid orange silver salt:
[00...
Embodiment 3
[0052] Synthesis of dodecyltriphenylphosphonium bromide:
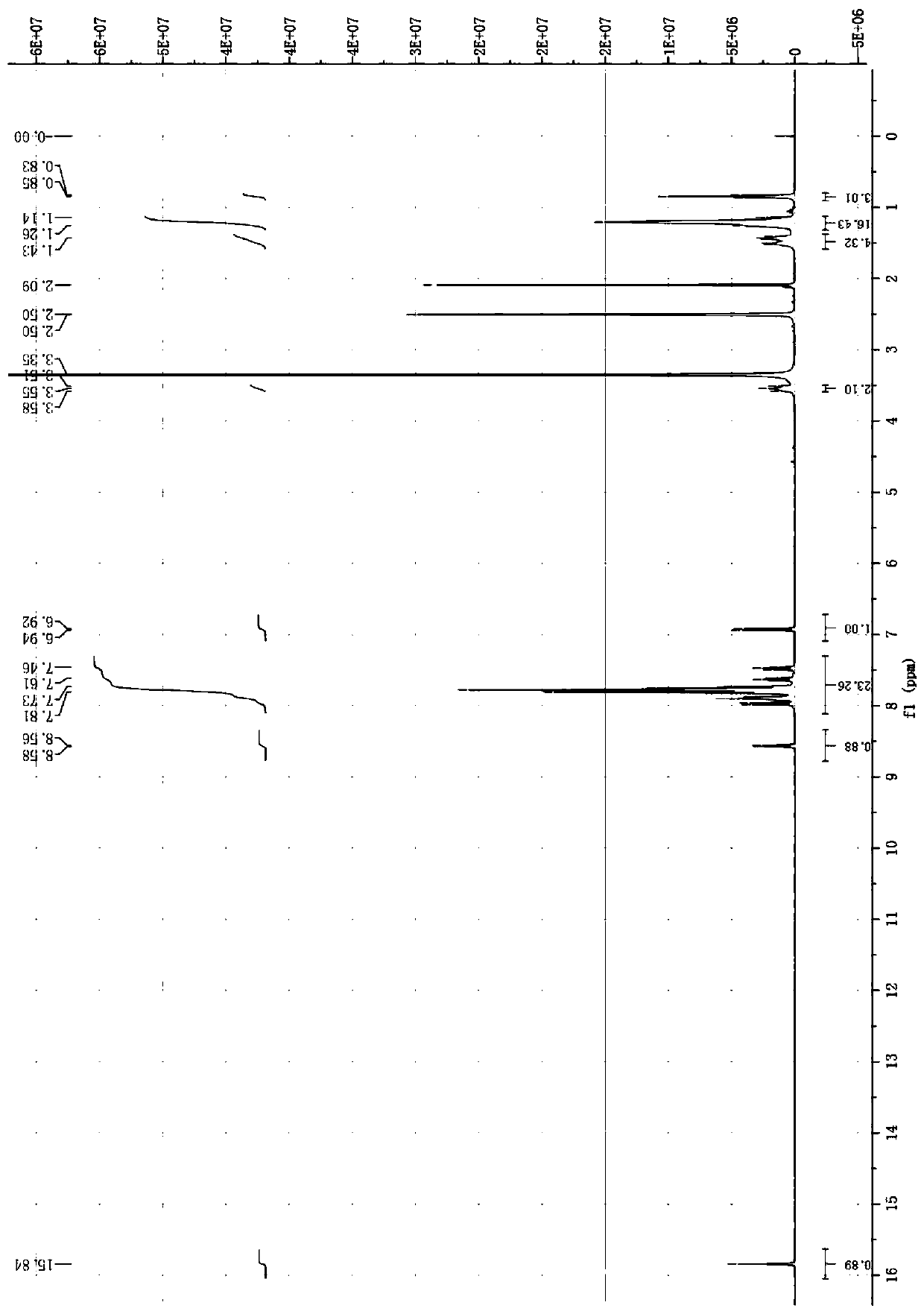
[0053] Add 1.31g (5.0mmol) of triphenylphosphine and 20mL of dry xylene into a 100mL three-necked flask, stir and dissolve at room temperature. Then, 1.38 g (5.5 mmol) of 1-bromododecane was slowly added dropwise while stirring. After the addition, the mixture was stirred and reacted at 120°C for 48 hours. After the reaction was completed, it was cooled to room temperature to obtain a viscous solid mixture. Add an appropriate amount of ethyl acetate and heat to 70°C, stir and wash thoroughly, then suction filter while it is hot, and repeat the washing 3 times to obtain a white solid, which is dried in vacuo to obtain 2.46 g of dodecyltriphenylphosphonium bromide as a white powder solid, with a yield of 96.1%.
[0054] 1 H NMR (400MHz, DMSO-d6) (ppm): δ=7.75-7.93 (m, 15H, H on the benzene ring), 3.54-3.62 (t, 2H, H on the methylene connected to P), 1.22 -2.51 (m, 20H, H on other methylene groups except the methylen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com