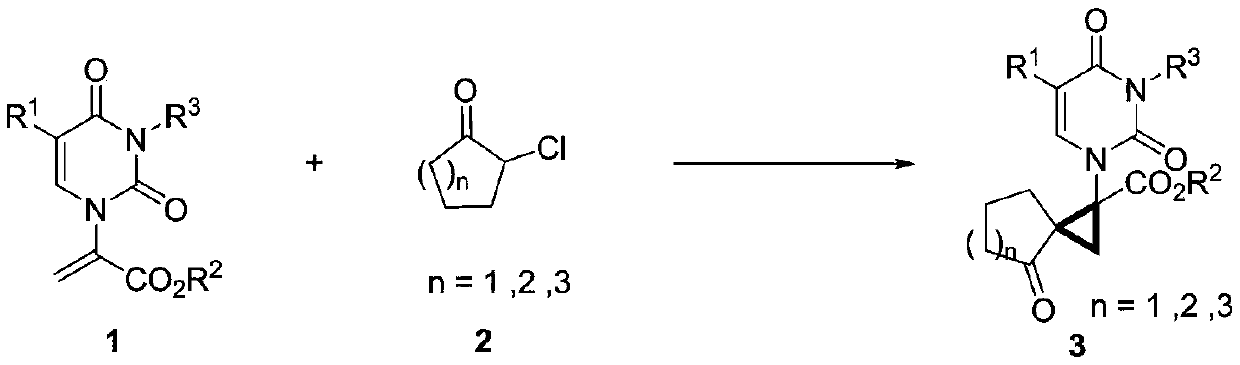
A method for synthesizing 2'-spirocyclyl substituted three-membered carbocyclic nucleosides
A three-membered carbocyclic and spirocyclic group technology, applied in the field of spirocyclic nucleoside synthesis, can solve the problems of increased reaction, long synthesis steps, and poor substrate universality, so as to achieve simple and easy-to-obtain raw materials, avoid numerous steps, The effect of rich product structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026]
[0027]
[0028] a Unless otherwise specified, the reaction steps are as follows: 1a (0.1 mmol), 2a (1.5 equiv), base (1.5 equiv) in air for 5 hours. b Separation yield c Potassium tert-butoxide (1.2 equiv) d Potassium tert-butoxide (2.0equiv) e 1h f 0.5h.
[0029] During the screening of reaction conditions, the effect of base on the reaction was first investigated (entries 1-6). Secondly, the solvent and temperature were investigated, and by comparing the yields, potassium tert-butoxide was finally determined as the optimal base, acetonitrile as the optimal solvent, and -20°C as the optimal temperature.
[0030] Typical reaction condition operation:
[0031] In a reaction flask, dissolve α-pyrimidine-substituted methyl acrylate 1a (0.1 mmol) and 1.5 eq (0.15 mmol) potassium tert-butoxide in 1 mL of acetonitrile, and place at -20°C for 10 minutes with stirring and cooling, then α-chloro Substituted cycloalkanone 2a (0.15 mmol) was added to the...
Embodiment 2
[0035] In a 10 mL vacuum tube, dissolve α-(3-benzoyl-5-methyl)uracil-substituted ethyl acrylate 1b and 1.5 eq (0.15 mmol) potassium tert-butoxide in 1 mL of acetonitrile and place at -20 °C for stirring After cooling for 10 minutes, α-chlorocycloalkanone 2a (0.15 mmol) was added to the reaction solution to react for 0.5 h. The reaction was monitored by TLC, quenched by adding water, and extracted with ethyl acetate. The combined organic phases were dried, filtered and concentrated in vacuo to give a crude oil. Then the target compound 3ba was obtained by column chromatography with a yield of 85%.
Embodiment 3
[0037] In a 10 mL vacuum tube, dissolve α-(3-benzoyl-5-methyl)uracil-substituted tert-butyl acrylate 1c and 1.5 eq (0.15 mmol) potassium tert-butoxide in 1 mL of acetonitrile and place at -20 °C Stir and cool for 10 minutes, then add α-chlorocycloalkanone 2a (0.15 mmol) into the reaction liquid for reaction, and react for 0.5 h. The completion of the reaction was monitored by TLC, quenched with water, and extracted with ethyl acetate. The combined organic phases were dried, filtered and concentrated in vacuo to give a crude oil. Then the target compound 3ca was obtained by column chromatography with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com