Application of licochalcone-A in preparation of anti-haemophilus-parasuis drug
A technology of Haemophilus suis and licorice chalcone is applied in the directions of antibacterial drugs, ketone active ingredients, etc., and can solve problems such as hindering the development and utilization of licorice chalcone A
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 In vitro inhibitory effect experiment of licochalcone A on Haemophilus parasuis
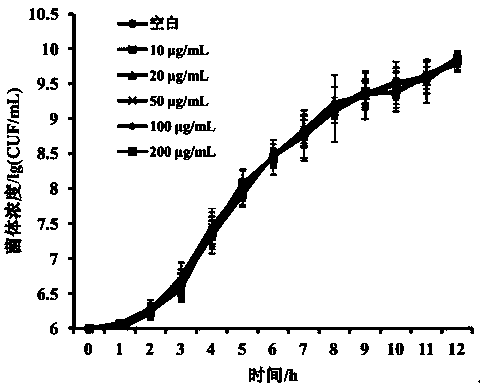
[0019] 1. Effect of licochalcone A on the growth rate of Haemophilus parasuis
[0020] The activated Haemophilus parasuis was inoculated in TSA liquid medium (containing newborn bovine serum and NAD), and cultured overnight at 37°C with shaking at 200r / min; then the bacterial liquid (10 6 CFU / mL) were respectively transferred to fresh liquid medium with final concentrations of 10 μg / mL, 20 μg / mL, 50 μg / mL, 100 μg / mL and 200 μg / mL licochalcone A, at 37° C culture, take out 100 μL of bacterial solution every 1 h and serially dilute it 10 times with sterile PBS, then take 100 μL of bacterial solution and spread it on the solid medium containing newborn bovine serum and NAD TSB, repeat 3 times for each dilution, and cultivate overnight After counting, draw the growth curve;
[0021] The effect of licochalcone A on the growth rate of Haemophilus parasuis as follows: figure 1 As s...
Embodiment 2
[0025] Example 2 The effect of licochalcone A on the growth and intestinal flora of mice
[0026] 1. Effect of licochalcone A on the growth of mice
[0027] 72 mice were randomly and equally divided into low-dose group, middle-dose group, high-dose group and control group. Low-dose group: Orally administered licochalcone A to mice at a dose of 1 mg / kg, 0.2 mL each time. Medium-dose group: Orally administered licochalcone A to mice at a dose of 10 mg / kg, 0.2 mL each time. High-dose group: Orally administered licochalcone A to mice at a dose of 50 mg / kg, 0.2 mL each time. Control group: normal feeding. The administration was administered by intragastric administration once a day for 7 consecutive days, the clinical symptoms of the mice were observed, the body weight of the mice was measured on the 3rd day, 5th day and 7th day respectively, and 6 mice were sacrificed respectively, and the lungs, heart, Kidney, thymus and spleen, calculate organ index, organ index = mouse orga...
Embodiment 3
[0037] Example 3 Inhibitory effect of licochalcone A on the proliferation of Haemophilus parasuis in mice
[0038] 1. The median lethal dose (LD) of Haemophilus parasuis to mice 50 ) determination
[0039] 72 mice were randomly divided into 6 groups (5 experimental groups and 1 blank group), and the freshly cultured Haemophilus parasuis was serially diluted 5-fold with sterile PBS, and an appropriate dilution was selected, and 200 μL / peritoneal Infect mice. Observe the state of the mice until there is no mouse death, record the death situation, and calculate the LD of Haemophilus parasuis on the mice according to the Bliss method 50 The results showed that the mice died on the 3rd day after the intraperitoneal injection of Haemophilus parasuis, and the death of the mice was continuously observed and recorded until no death occurred within 5 days. The LD50 of bacillus H12L1 to mice was 8.72×10 7 CFU / mL.
[0040] 2. Effect of licochalcone A on the proliferation of Haemophil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com