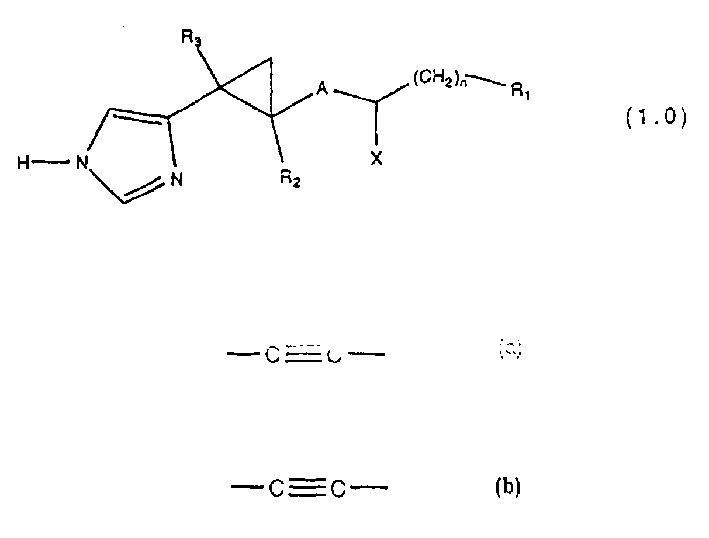
2-(1H-4(5)-imidazoyl cyclopropyl derivatives
A kind of composition and compound technology, applied in the field of treating diseases that need to block histamine H3 receptors, 1H-4-substituted imidazole derivatives and salts or solvates thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] N-(1-benzyl)-3-[(1H-imidazol-5-yl)]-2(R)-3(S)-cyclopropanamide and N-(1-benzyl)-3-[( Preparation of 1H-imidazol-5-yl)]-2(S)-3(R)-cyclopropanamide hydrochloride racemic mixture
[0136] According to Burger et al., J. Med. Chem., (1970), 13 : 3-[(1-triphenylmethyl-5-imidazolyl)]-2(R)-3(S)-cyclopropionic acid and 3-[(1-triphenyl) prepared by the method described in 33-35 The racemic mixture (0.334 g, 0.84 mM) of 2(S)-3(R)-cyclopropionic acid) was suspended in 5 mL of distilled water. Sufficient acetone (35 mL) was added throughout the solution and the homogenized solution was cooled to 0-5°C. Triethylamine (0.101 g, 1.0 mM) in 5 mL of acetone was added, followed by the dropwise addition of ethyl chloroformate (0.108 g, 1.0 mM). The reaction mixture was stirred at 0 °C for 30 minutes, then benzylamine (0.16 g, 1.5 mM) in 10 mL of acetone was added dropwise. The reaction mixture was stirred at 0-5°C for 1 hour, then cold saturated ammonium chloride solution (100 mL) wa...
Embodiment 2
[0141] N-(1-cyclohexylmethyl)-3-(1H-imidazol-5-yl)-2(R)-3(S)-cyclopropanamide and N-(1-cyclohexylmethyl)-3- Preparation of (1H-imidazol-5-yl)-2(S)-3(R)-cyclopropanamide hydrochloride racemic mixture
[0142] Except that benzylamine is replaced with cyclohexanemethylamine, the method described in Example 1 is followed to obtain N-(1-cyclohexylmethyl)-3-(1H-imidazol-5-yl)-2(R) -3(S)-cyclopropanamide and N-(1-cyclohexylmethyl)-3-(1H-imidazol-5-yl)-2(S)-3(R)-cyclopropanamide hydrochloride racemic mixture. NMR (CD 3 OD, 300MHz): d7.78(s, 1H), 6.92(s, 1H), 3.02(m, 2H), 2.30(m, 1H), 1.84(m, 1H), 1.74(m, 4H), 1.45 (m, 1H), 1.35(m, 1H), 1.22(m, 3H), 0.94(m, 2H) mass spectrum (DCl / NH 3 ): 248(M+1) + , MW=247.3422, C 14 h 21 N 3 o 1 .
Embodiment 3
[0144] N-[1-(3-aminopropyl)-2-methylpiperidine]-3-(1H-imidazol-5-yl)-2(R)-3(S)-cyclopropanamide and N-[ 1-(3-aminopropyl)-2-methylpiperidine]-3-(1H-imidazol-5-yl)-2(S)-3(R)-cyclopropanamide hydrochloride racemic mixture preparation of
[0145] Except that 1-(3-aminopropyl)-2-methylpiperidine is used instead of benzylamine, the method described in Example 1 is followed to obtain N-[1-(3-aminopropyl)-2-methylpiperidine Basepiperidine]-3-(1H-imidazol-5-yl)-2(R)-3(S)-cyclopropanamide and N-[1-(3-aminopropyl)-2-methylpiperidine ]-3-(1H-Imidazol-5-yl)-2(S)-3(R)-cyclopropanamide hydrochloride racemic mixture. NMR (CD 3 OD, 300MHz): d8.80(s, 1H), 7.38(s, 1H), 3.55(m, 1H), 3.3(m, 3H), 3.1(m, 3H), 2.44(m, 1H), 1.96 (m, 4H), 1.8(m, 2H), 1.55(m, 2H), 1.39(d, 3H, J=6Hz), 1.35(m, 4H) mass spectrum (DCl / NH 3 ): 291 (M+1) + , MW=290.4109, C 16 h 26 N 4 o 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com