An analytical method for multiple active ingredients in oral contraceptive placebo tablets
A technology of active ingredients and analysis methods, applied in the field of analysis, can solve the problems of simultaneous detection of active ingredients, etc., and achieve the effect of simple testing and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
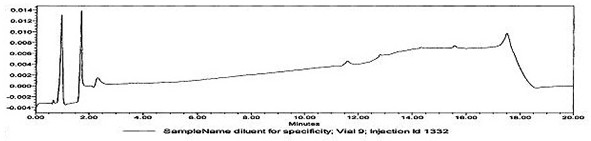
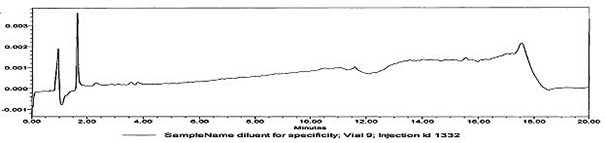
[0030] Determination of whether multiple active ingredients are contained in oral contraceptive blank tablets:
[0031] The tablet shall not contain the active ingredients of oral contraceptives: norgestimate, levonorgestrel, norethindrone acetate, norethindrone and ethinyl estradiol.
[0032] (1) Instruments and conditions
[0033] Liquid chromatograph: high performance liquid chromatography system and workstation;
[0034] Chromatographic column: Octadecylsilane bonded silica gel as filler, column length 150mm, inner diameter 4.6mm, particle size 5mm;
[0035] Detection wavelength: 210nm and 245nm
[0036] Injection volume: 50µL
[0037] Column temperature: 40°C
[0038] Analysis time: 18 minutes
[0039] gradient:
[0040]
[0041] (2), experimental steps
[0042] Preparation of mobile phase A (volume ratio of acetonitrile to water is 22:78):
[0043] Measure 440 ml of acetonitrile and 1560 ml of water in a measuring bottle, shake well.
[0044] Preparation of m...
Embodiment 2
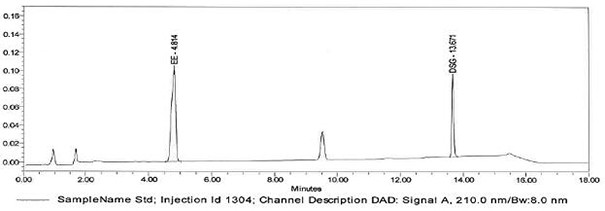
[0056] Determination of whether multiple active ingredients are contained in oral contraceptive blank tablets:
[0057] The tablet shall not contain the active ingredients of oral contraceptives: norgestimate, levonorgestrel, norgestimate, norethindrone acetate, norethindrone, drospirenone, desogestrel and ethinyl estradiol.
[0058] (1) Instruments and conditions
[0059] Liquid chromatograph: high performance liquid chromatography system and workstation;
[0060] Chromatographic column: Octadecylsilane bonded silica gel as filler, column length 150mm, inner diameter 4.6mm, particle size 5mm;
[0061]Detection wavelength: 210nm and 245nm
[0062] Injection volume: 50µL
[0063] Column temperature: 40°C
[0064] Analysis time: 18 minutes
[0065] gradient:
[0066]
[0067] (2), experimental steps
[0068] Preparation of mobile phase A (volume ratio of acetonitrile to water is 22:78):
[0069] Measure 220 ml of acetonitrile and 780 ml of water in a measuring bottle,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com