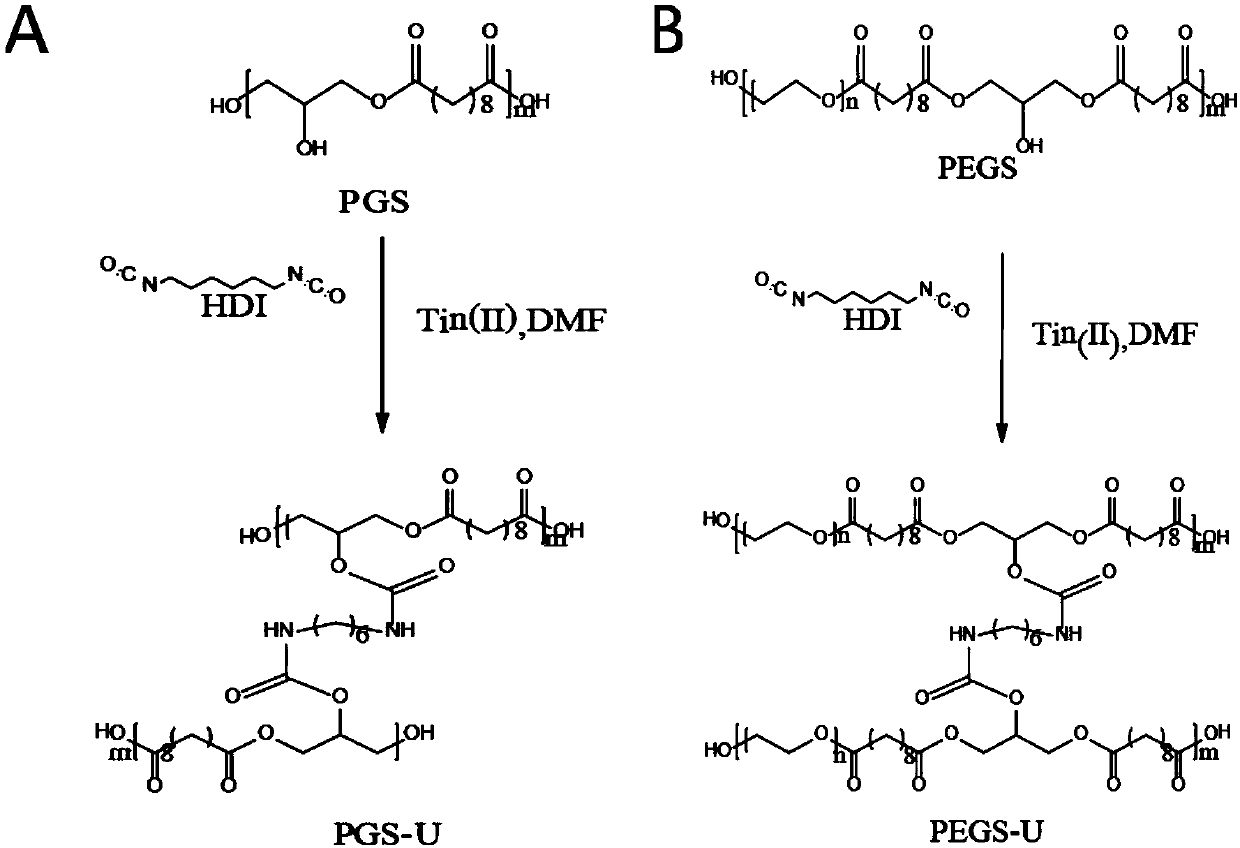
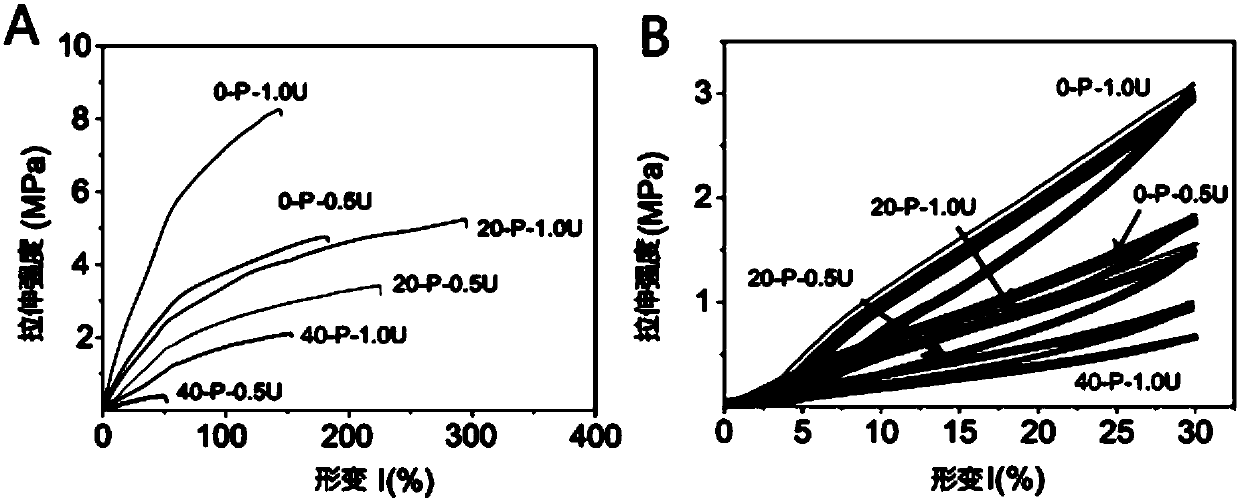
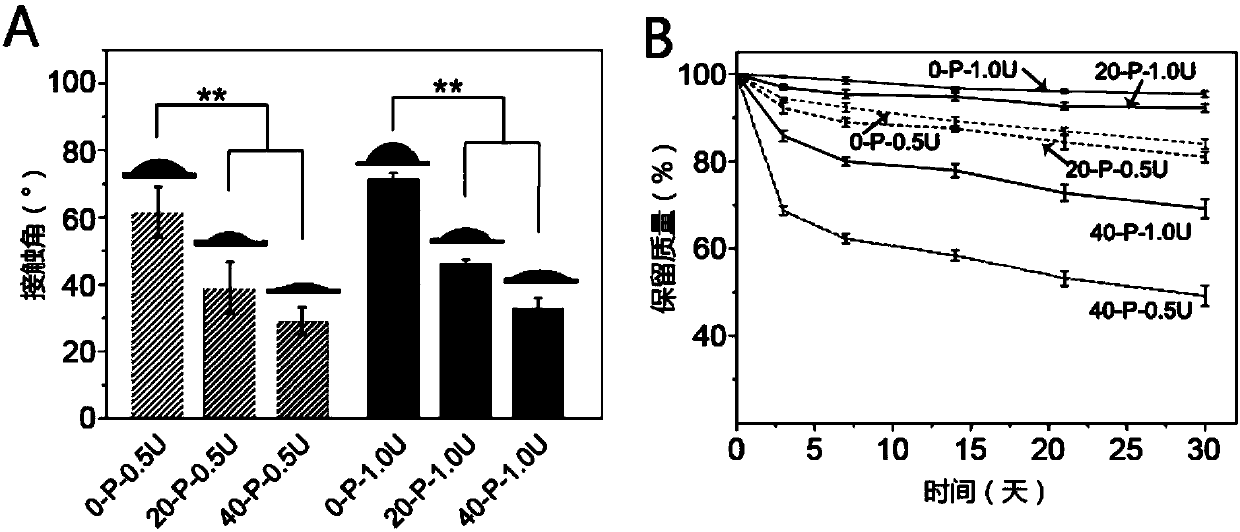
Isocyanate crosslinked polyethylene glycol-polyglycerol sebacate bio-elastomer as well as preparation method and application thereof
A technology of polyglyceryl sebacate and polyethylene glycol, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the problems of poor hydrophilicity, complex cross-linking conditions of polyglyceryl sebacate, and soft tissue use Limited performance and other issues, to achieve the effect of promoting regeneration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] This embodiment relates to the synthesis and purification of PEGSO
[0103] PEGSO prepolymers are synthesized by a two-step method:
[0104] (a) Under an argon atmosphere, sebacic acid and glycerin with a molar ratio of 1:1 were reacted at 130° C. for 24 hours;
[0105](b) The product of step a) was continued to react for 6 hours at 150°C under vacuum conditions to obtain a PEGSO prepolymer.
[0106] The prepolymerized product is purified through repeated ethanol dissolution-water sedimentation operations to remove unreacted monomers and small molecules to obtain purified PEGSO prepolymer (polyglyceryl sebacate). The number average molecular weight was 6023 Da, and the dispersibility coefficient was 3.21.
Embodiment 2
[0108] This embodiment relates to the synthesis and purification of PEGS20 (20% polyethylene glycol-polyglyceryl sebacate)
[0109] (i) under an argon atmosphere, 14.14g of sebacic acid and 20.00g of PEG (the number average molecular weight is 1000g / mol) were reacted at 130°C for 2 hours;
[0110] (ii) continue to react the product of step (a) for 24 hours at 130° C. under vacuum conditions to obtain a linear prepolymer of sebacic acid and PEG;
[0111] (iii) Add 7.36 g of glycerin and 14.14 g of sebacic acid to the product of step (b), and continue to react at 130° C. under vacuum for 48 hours. The molar content of PEG relative to glycerol in this reaction was 20%, and the total reacted hydroxyl to carboxyl molar ratio was 1.
[0112] The prepolymerization product is purified through repeated ethanol dissolution-water precipitation operations to remove unreacted monomers and small molecules to obtain a purified PEGS20 prepolymer. The actual PEG content was 19%, the number a...
Embodiment 3
[0114] This embodiment relates to the synthesis and purification of PEGS80 (80% polyethylene glycol-polyglyceryl sebacate)
[0115] (a) under an argon atmosphere, 16.18g of sebacic acid and 80.00g of PEG (the number average molecular weight is 1000g / mol) were reacted at 130°C for 2 hours;
[0116] (b) continue to react the product of step (a) for 24 hours at 130° C. under vacuum conditions to obtain a linear prepolymer of sebacic acid and PEG;
[0117] (c) Add 1.8414 g of glycerin and 6.0675 g of sebacic acid to the product of step (b), and continue to react at 130° C. under vacuum for 48 hours. The molar content of PEG relative to glycerol in this reaction was 80%, and the molar ratio of hydroxyl and carboxyl groups in the total reaction was 1:1.
[0118] The prepolymerized product is purified through repeated ethanol dissolution-water sedimentation operations to remove unreacted monomers and small molecules to obtain a purified PEGS80 prepolymer. The actual PEG content is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mechanical strength | aaaaa | aaaaa |
| Mechanical strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com