Indole synthase and its application
A technology for terpenes and sesquiterpenes, which is applied to terpenoid synthase and its application field, can solve the problems of increasing the difficulty of sesquiterpene skeleton and limiting the diversity of skeleton.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] In this example, the cDNA sequence of terpene synthase J1-018-A (abbreviated as J1-018-A) was amplified by PCR method, and the cDNA sequence was used to construct a plasmid and a high-yield sesquiterpene yeast platform, wherein, The strains and plasmids used in this example are shown in Table 1, and the primers used to construct related plasmids are shown in Table 2.
[0059] The main characteristics of the strains and plasmids used in Table 1
[0060]
[0061] Table 2 Primers used for plasmid construction
[0062]
[0063]
[0064]
[0065] 1. J1-018-A plasmid construction and protein purification
[0066] Using the reverse-transcribed F. graminearum J1-012 cDNA as a template, use primers P1 / P2 to amplify to obtain the cDNA sequence of J1-018-A, and then enzyme-digest and connect it to plasmid pET28a(+) to obtain plasmid pGB152, as follows:
[0067] (1) Obtaining cDNA: QIAGEN RNeasy Plant Mini Kit was used for fungal RNA extraction, and the operation was ...
Embodiment 2
[0117] In this example, the J1-018-A fermentation product obtained in Example 1 is purified and identified, as follows:
[0118] 1. Product purification of S.cerevisiae T16 mutant strain
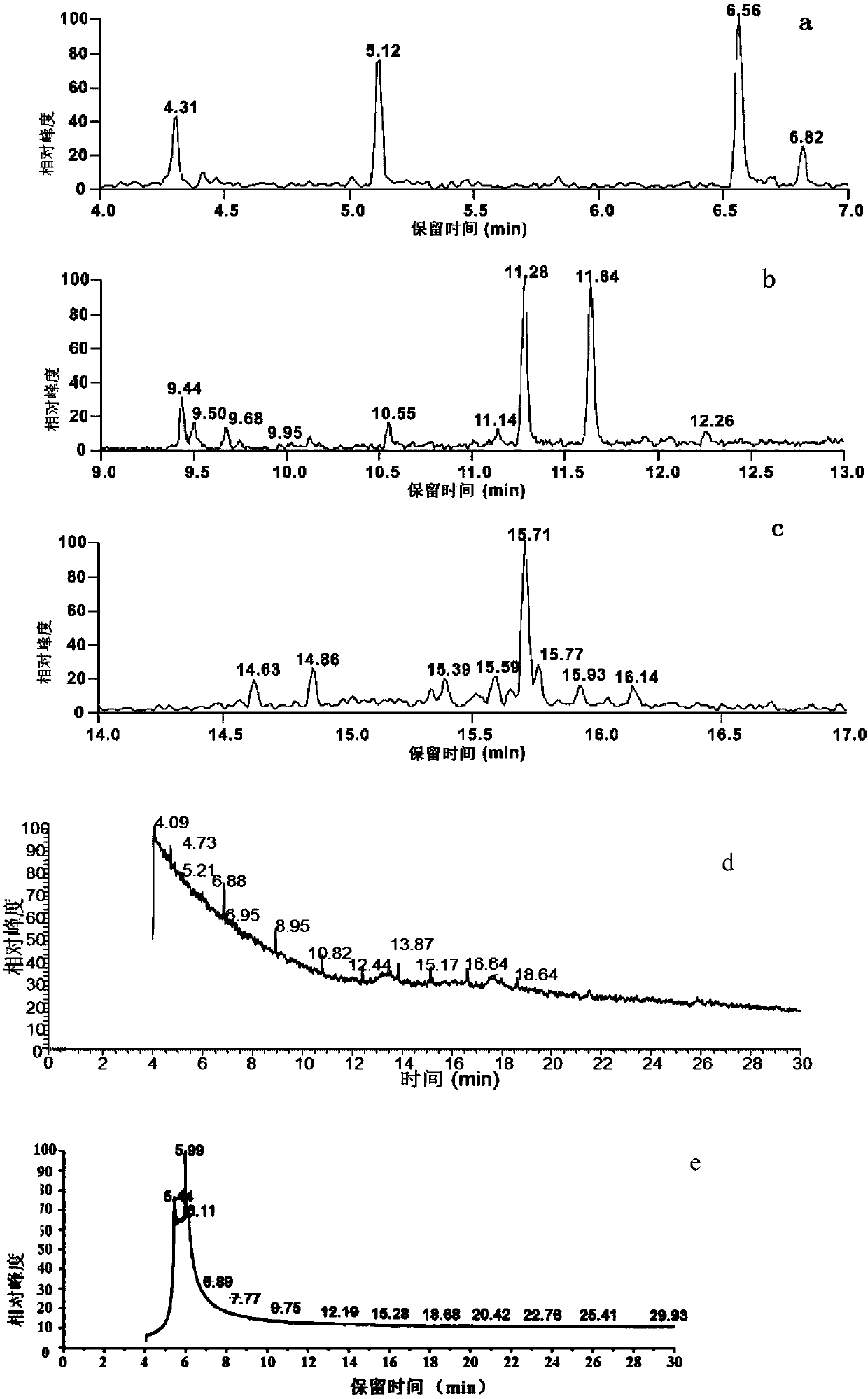
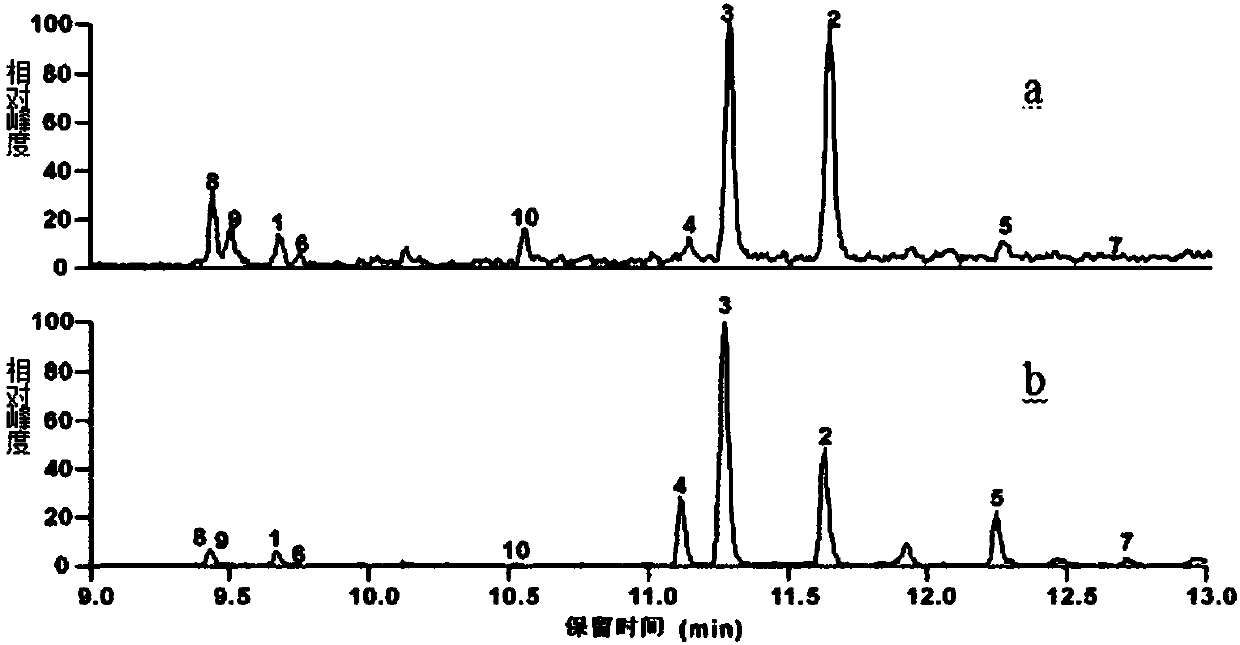
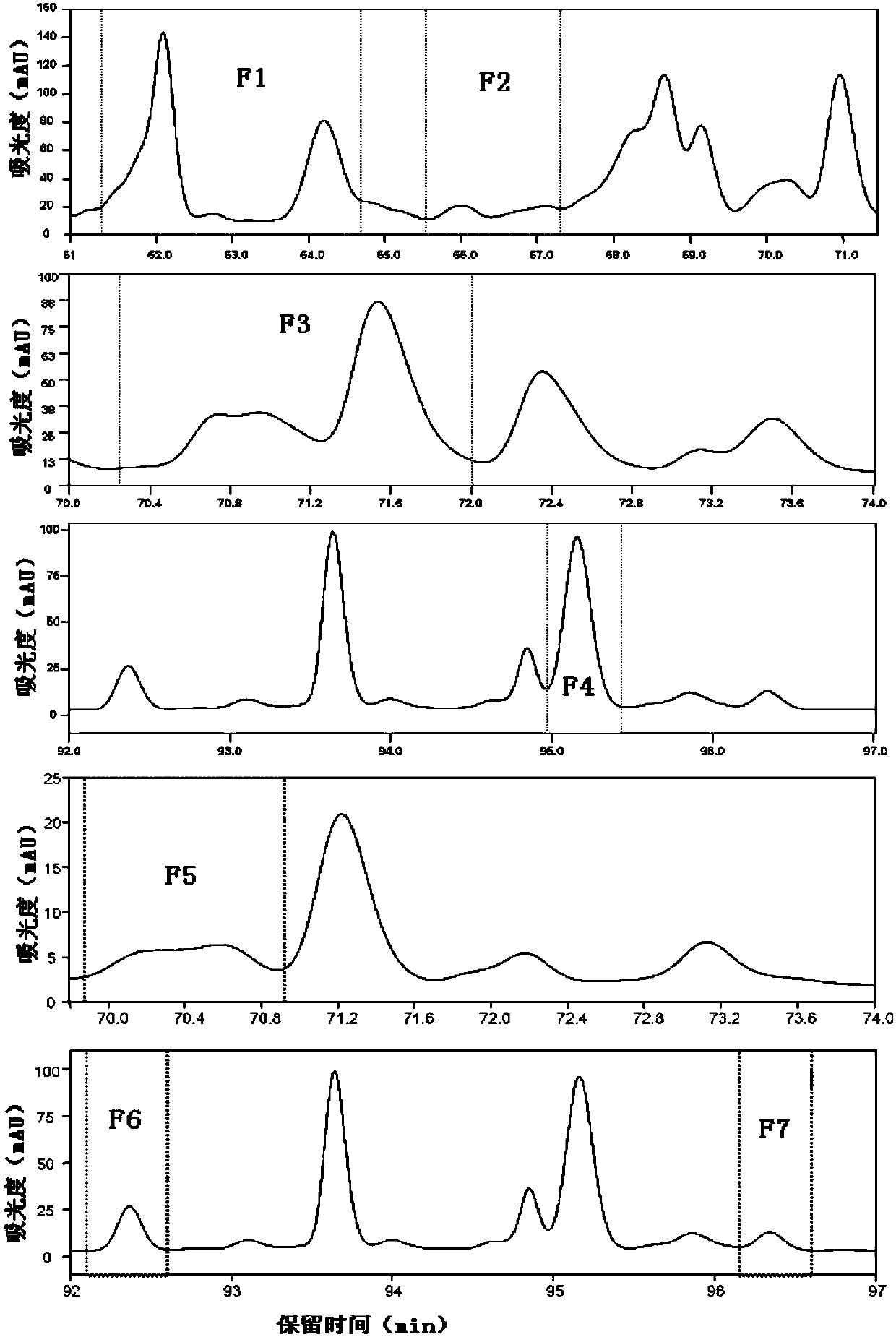
[0119] Purified mobile phase A is ultrapure water, mobile phase B is acetonitrile, and the ultraviolet absorption wavelength is 210nm. The J1-018-A fermentation product is purified by semi-preparative HPLC according to the conditions described in Table 1, and the target product is determined by GC-MS According to the peak time on HPLC, the F1-F7 components containing sesquiterpene products were collected, among which, F1-F4 were the main sesquiterpene components. For details, see image 3 .
[0120] Purification conditions of compounds in table 1S.cerevisiae T16 mutant strain
[0121]
[0122] The analysis results show that the purity of compound 1 in component F4, compound 6 in component F6 and compound 8 in component F7 can be directly used for NMR detection without further purificati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com