Cell perfusion culture method for mini model based on Tubespin bioreactor
A technology of bioreactor and perfusion culture, which is applied in the field of cell perfusion culture of reduced models, can solve the problems of high cost and insufficient parallelism, and achieve the effects of low cost, easy operation and reduced shearing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The cell perfusion culture method based on the reduced model of Tubespin bioreactor of the present embodiment comprises the following steps:
[0050] (1) Expansion of seed cells: CHO cells stably expressing monoclonal antibody mAb are amplified in ActiPro containing 4mM Gln after recovery, passed once every three days, and placed in a 125-250mL conical shaker flask for culture and expansion. 37℃, rotation radius 25mm, speed 110rpm, humidity 80%, 5% CO 2 Infors shaker, when the cells grow to (3.0-4.0) × 10 6 cells / mL for next inoculation;
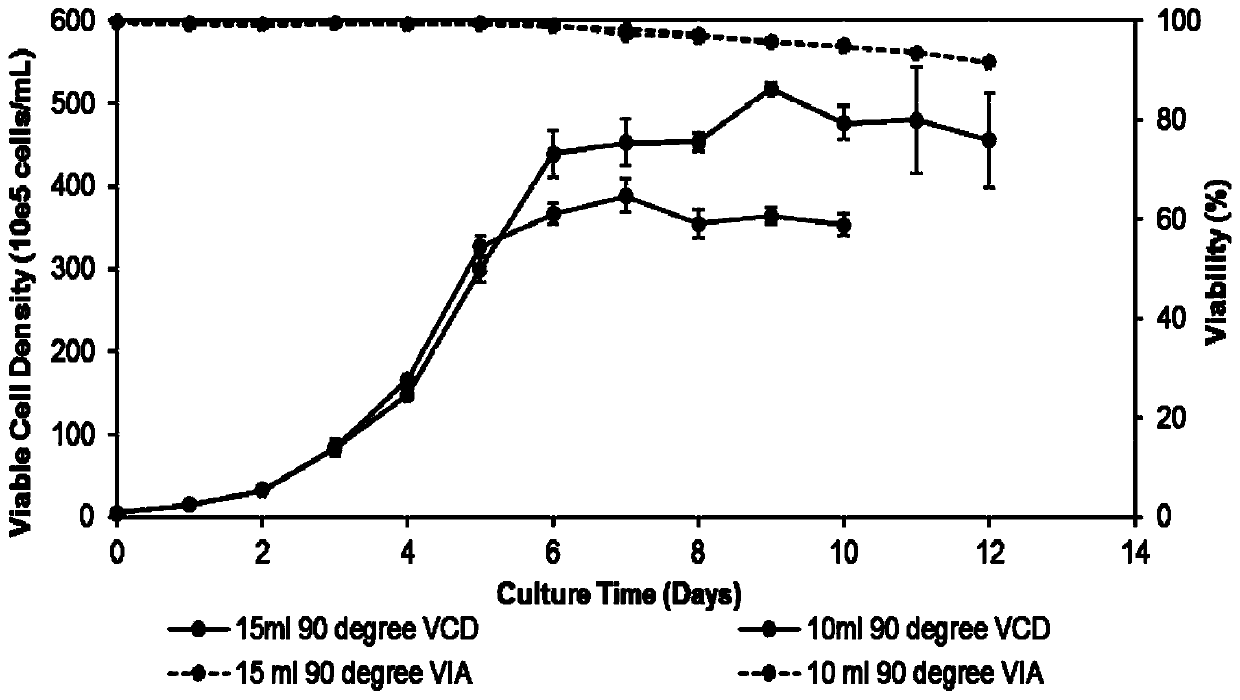
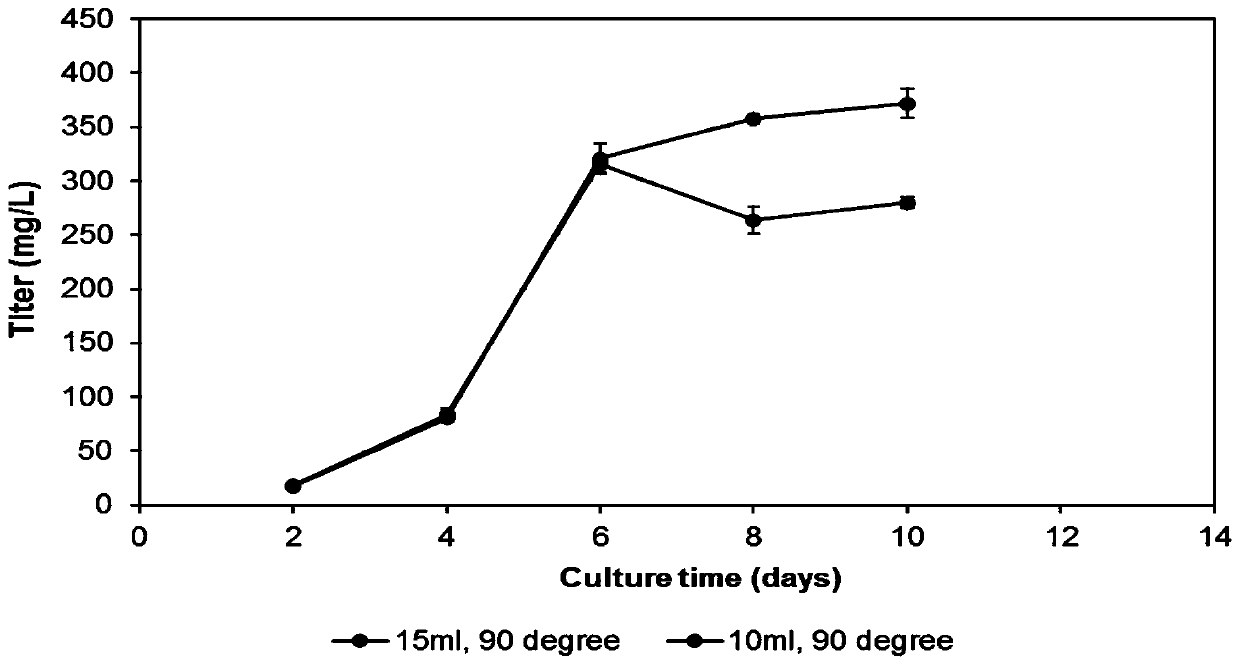
[0051] (2) The cells obtained in step (1) were divided into 0.5×10 6 cells / mL were inoculated into a 50mL Tubespin bioreactor, the volume of the cell liquid was 10mL, the angle of the Tubespin bioreactor was adjusted to 90° (the Tubespin bioreactor was perpendicular to the horizontal plane of the shaker), and no anti-shearing agent was added; Tubespin biological The reactor is cultivated at 37°C, the rotation radius is 50mm, the ro...
Embodiment 2
[0054] The cell perfusion culture method based on the reduced model of Tubespin bioreactor of the present embodiment comprises the following steps:
[0055] (1) Expansion of seed cells: CHO cells stably expressing monoclonal antibody mAb were amplified in ActiPro containing 4mMGln after recovery, passed once every three days, and placed in a 125-250mL Erlenmeyer shaker flask at 37 ℃, rotation radius 25mm, speed 110rpm, humidity 80%, 5% CO 2 Infors shaker, when the cells grow to (3.0-4.0) × 10 6 cells / mL for next inoculation;
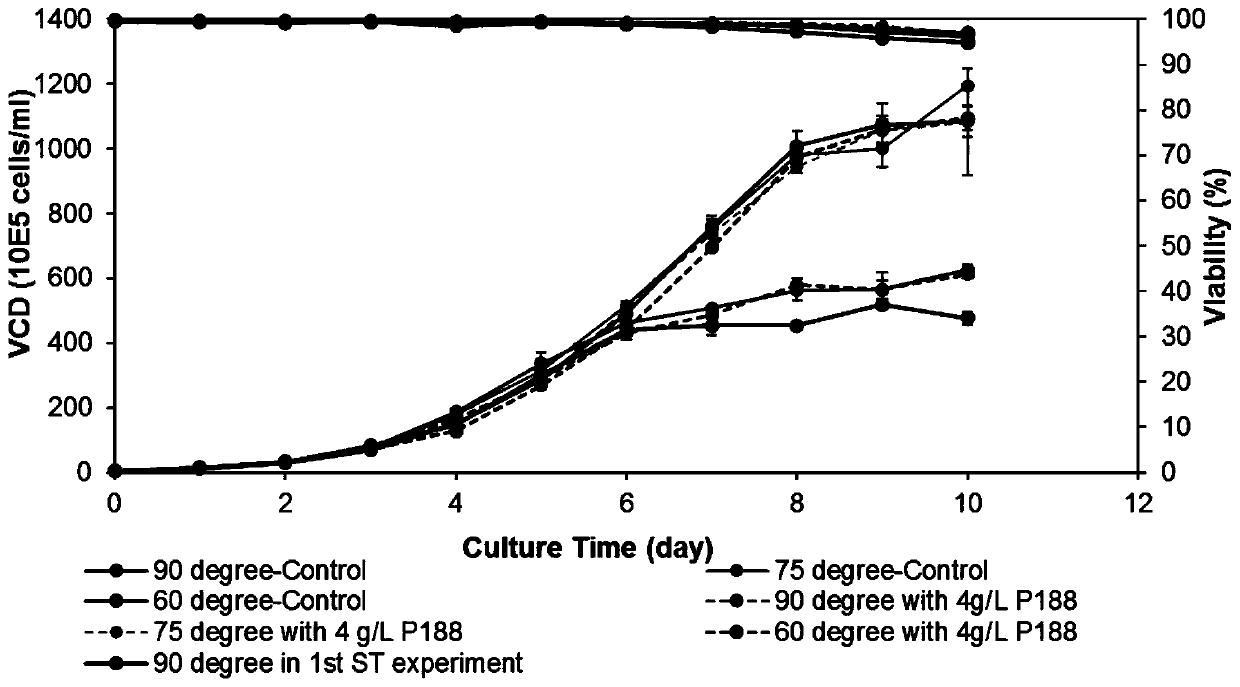
[0056] (2) The cells obtained in step (1) were divided into 0.5×10 6 Inoculate the cells / mL into a 50mL Tubespin bioreactor, the cell liquid volume is 15mL, adjust the angle of the Tubespin bioreactor tube stand to 90° (the Tubespin bioreactor is perpendicular to the horizontal plane of the shaker), and do not add anti-shearing agent; Tubespin bioreactor culture at 37°C, rotation radius 50mm, rotation speed 190rpm, humidity 80%, 5% CO 2 Infors shaker...
Embodiment 3
[0059] The cell perfusion culture method based on the reduced model of Tubespin bioreactor of the present embodiment comprises the following steps:
[0060] (1) Expansion of seed cells: CHO cells stably expressing monoclonal antibody mAb are amplified in ActiPro containing 4mM Gln after recovery, passed once every three days, and placed in a 125-250mL conical shaker flask for culture and expansion. 37℃, rotation radius 25mm, speed 110rpm, humidity 80%, 5% CO 2 Infors shaker, when the cells grow to (3.0-4.0) × 10 6 cells / mL for next inoculation;
[0061] (2) The cells obtained in step (1) were divided into 0.5×10 6 cells / mL were inoculated into a 50mL Tubespin bioreactor, and the volume of the cell liquid was 10mL. Adjust the angle of the Tubespin bioreactor to 60° (60° between the Tubespin bioreactor and the horizontal plane of the shaker), without adding anti-shear reagent; Tubespin bioreactor culture at 37 ℃, rotation radius 50mm, rotation speed 190rpm, humidity 80%, 5% C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com